Abstract
Objective
The aim of this study was to evaluate the impact of patent expiry on drug prices by means of a systematic literature review.
Methods
A systematic literature search was performed in PubMed to identify all published literature on the impact of patent expiration on drug prices. Additional literature was identified using a less distinct syntax in Google Scholar and EconLit. Data extraction followed a standardized assessment form containing the domains study type, study aim, reported outcomes, number of drugs and drug classes assessed, and originators or generics assessed.
Results
The 16 identified studies that assessed impact of patent expiry on drug prices showed that price developments after patent expiration varied between countries. The included studies assessed price developments for the USA, Canada, Australia, the UK, the Netherlands, Germany and France, Spain, Italy, Norway, Sweden and Denmark. The number of drugs included within different studies ranged between 1 and 219. The identified studies indicated that drug prices decreased significantly after patent expiry with drug price ratios ranging from 6.6 to 66% 1–5 years after patent expiry.
Conclusion
Drug prices decrease significantly after patent expiry. The extent of this price reduction varied greatly between products and countries. For this reason, country-specific analyses on price developments after patent expiry should be used when these are considered in decision making. Future research should be dedicated to gathering more country-specific data to reduce the uncertainty with regard to price developments.
Similar content being viewed by others
Drug prices decrease significantly after patent expiry. |
Country-specific data on drug price developments is lacking for the European market. |
Country-specific data on drug price developments should be used in decision making where drug prices play a prominent role. |
1 Introduction
In high-income countries, total health expenditure represented 12.3% of the gross domestic product (GDP) in 2014 [1]. Following the great recession of 2008, health expenditures have once again become a major target of cost-containment efforts at national level [2]. In the Netherlands, the total healthcare expenditure rose from €67 billion in 2005 to €96 billion in 2016. The budget spend on pharmaceuticals during the same time period remained rather stable though, and accounted for approximately 10% of these healthcare expenditures [3]. Though newly introduced pharmaceuticals may pressure a given healthcare budget, patent expiration and associated price decreases may offset this burden. After a patent expiry or loss of other exclusivity rights, generic copies of the originator can be produced and marketed without a license from the originator company [4]. The market entry of generic copies of originator drugs after patent expiry and subsequent generic substitution play a role in the cost containment in health care and pharmaceuticals [5].
Patents can foster innovation as they provide the manufacturer the opportunity for a temporary monopoly and a period of market exclusivity [6]. During the period of market exclusivity pharmaceutical companies can recoup the opportunity costs made during the drug development process. The right of market exclusivity for new products stimulates new investments in Research and Development (R&D). Several factors may influence the duration of market exclusivity, including: the moment of patent filing, the duration of the R&D process afterwards, the registration process and time to approval/reimbursement by the US Food and Drug Administration (FDA)/European Medicine Agency (EMA) and national health technology assessment (HTA) agencies, and the duration before approval of generic drugs [7].
The aim of this study was to evaluate available quantitative data regarding impact of patent expiry on drug prices by means of a systematic literature review.
2 Methods
2.1 Literature Search
A systematic literature search was performed on peer-reviewed literature in the PubMed, EconLit and Google Scholar databases in February 2018. A comprehensive search syntax that included the terms “patent”, “brand”, “licensed”, “market exclusivity”, “drug”, “medicine”, “pharmaceutical”, “expiration”, “expiry”, “generic entry”, “generic substitution”, “lifecycle”, “originator”, “price”, “cost”, “cost effectiveness”, “ICER” was run through the PubMed Database (Appendix 1). A subsequent literature search was performed using a less distinct syntax with the terms “generic substitution” and “patent expiry” for titles and abstracts in the EconLit database and Google Scholar. Additionally, the references in the bibliography of the papers selected from the included databases were reviewed manually. Searches were limited to publications from the year 2000 onwards.
2.2 Literature Selection
The selected literature was limited to full publications of original research. Studies were included if they reported quantitative outcomes on the impact of patent expiration on drug prices. Exclusion criteria included: (a) papers were not written in English or Dutch, or (b) the endpoints (reported outcomes) on price developments did not include or were not comparable to moment of initial generic entry. Reported endpoints on price developments after patent expiry were extracted as outcome for this study. The selection of literature and data extraction was performed by two authors (GTV and QC). In case of disagreement, a third author was consulted(MHR).
2.3 Data Analysis
A standardized assessment form was developed to extract data, which included the following domains: study type, study aim, reported outcomes and results, number of drugs assessed, drug class, originators or generics assessed, and time point after patent expiry of reported price development outcomes. The moment of patent expiry was defined as the moment of initial generics entry. A generic drug was defined as a pharmaceutical drug that is equivalent to a brand-name product in route of administration, quality, performance, and intended use. Reported outcomes on price developments were translated into price ratios compared to the price of the original product at moment of patent expiry, where necessary.
3 Results
3.1 Results of Data Extraction
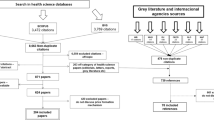
The systematic PubMed search yielded 262 unique citations. After screening for titles and abstracts 214 citations were excluded based on the exclusion criteria. The remaining 48 articles underwent full-text examination for eligibility, which resulted in the inclusion of ten articles [8,9,10,11,12,13,14,15,16,17]. The remaining 38 articles were excluded as they examined policy changes, prescription patterns, or total healthcare expenditures rather than price developments after patent expiry. The literature search in the EconLit database yielded 36 articles of which 11 were deemed eligible for full-text examination. Seven articles met the inclusion criteria of which two were duplicates from the PubMed search [18,19,20,21,22,23,24]. One additional study that met the selection criteria was identified in the literature search in Google Scholar [6]. As a result, in total 16 studies were selected for data extraction (Fig. 1).
Table 1 summarizes the included studies in this review. Studies focused on 12 different countries over three continents. The majority of the studies focused on or included the USA (n = 9) [6, 8, 10, 11, 15,16,17,18, 21, 22, 24]. Furthermore, one study focused on Canada [14], one study on the Netherlands [12], one on Spain [20], and four studies assessed the price developments in multiple countries [9, 13, 19, 23]. In total 16 studies examined the impact of patent expiration on drug list prices over time [6, 8,9,10,11,12,13,14,15, 18,19,20,21,22,23,24].
3.2 Time Period and Number of Drugs Included
Eleven studies identified and assessed all drugs facing initial generic entry within their study period, to provide a representative overview of the impact of patent expiry on price for the total market [6, 8, 10, 11, 13,14,15, 18, 20, 21, 24] (see also Tables 1 and 2). The remaining five articles focused on a specific therapeutic area or specific drugs [19, 22, 23].
All studies included in this review assessed price developments of between one and 129 different drugs. Price developments of the included drugs were assessed between 1984 and 2009, and the average study period length was 7.5 years [8].
3.3 Definition of Patent Expiry
As the moment of patent expiration varies between countries, and drugs are often protected by several different patents, it can be challenging to define a set point for patent expiry. Notably, studies included in this review that assessed the impact of expiry on drug prices used varying definitions for the set time of patent expiry. Seven studies used the time of initial generic entry as a surrogate endpoint for the moment of patent expiry [8, 10, 11, 14, 20, 22], whereas in six the date of patent expiry was available within the used database (MIDAS-IMS International database and the IMS Generic Spectra database) [6, 13, 19, 21, 23, 24]. The remaining three studies that assessed the impact of expiry dates on drug prices did not specify how the patent expiry date was set [9, 12, 15].
3.4 Outcome Measurement and Data Use
Various outcome measures were used in the studies included in this review. Whereas seven studies assessed the impact of generic entry on both originator and generic prices [6, 15, 18, 19, 21, 22, 24], five studies only reported generic price developments [8,9,10, 12, 20], and four studies focused only on price developments of originator drugs after generic entry [11, 13, 14, 23].
Furthermore, 12 studies defined their outcome as a price ratio compared to the originator price at the moment before patent expiry (seven for both generic and originator drugs, three for generics only and three for originators only) [6, 8, 10, 13,14,15, 18, 20,21,22,23,24], one study reported the outcome as wholesale price per tablet [9], and two studies used price per Defined Daily Dose (DDD) as the comparative outcome measurement [12, 18]. In addition, two studies reported on the price rigidity of originator drugs and reported price changes in percentages at certain time points after patent expiry [11, 23].
The type of data used to estimate the impact of patent expiry on drug prices also varied between studies. Thirteen studies used drug-utilization data, which allowed for the correction of the proportionate use of different strengths and administration forms of both originators and generics [6, 8,9,10, 12, 13, 15, 18, 19, 21,22,23,24], whereas three other studies only used price data [11, 14, 20]. A further three studies used the assumed average maintenance dose per day for a drug (DDDs) as a comparative measure [12, 13, 15], and three more used real-world prescription or utilization data for the investigated drugs to weight prices [6, 8, 22]. Lastly, the remaining two studies did not elaborate on how utilization data was used to weight prices [9, 10].
3.5 Outcomes
The 12 studies that assessed generic prices after patent expiry showed price ratios ranging from 6.6% up to 66% after 1–5 years after initial generic entry (see Table 2) [6, 8,9,10, 12, 15, 18,19,20,21,22, 24]. Both Berndt et al. [10] and Wiggins et al. [22] additionally detected an inverse relation between the number of generic entrants and the drug price, although beyond five generic entrants no further impact on the drug price was observed [10]. Similarly, Kelton et al. showed that for every additional generic introduced, the relative reimbursement price of a drug would decrease with 13% on average [8].
Of the six studies that assessed the impact of patent expiration on originator prices [6, 11, 13,14,15, 23], the four studies that focused on the USA concluded that originator prices were rigid and overall did not alter significantly after patent expiry [6, 11, 14, 15]. However, both the studies by Magazzini et al. and Vandoros et al. showed that the impact of patent expiration varied between different countries [13, 23]. For example, Magazzini found that in France the price after patent expiry was rigid for originators, while in Germany and the UK originator prices decreased by 25% on average in the 9 months after patent expiry, and in the USA originator prices increased by 10–20% over the same period [13]. Based on the analyses in Denmark, Germany, Norway, Sweden, the UK, and the Netherlands, Vandoros reported that overall originator prices were 5% higher in markets where generic counterparts were available. In Denmark and Sweden originator prices were 2.5 and 0.8% lower when generics were available, while in Germany, Norway, the UK and the Netherlands originator prices were found to be 1.3, 3.8, 4.1 and 11.3% higher, respectively, for products with generics available. Country differences were also analyzed by Clarke et al. who showed large differences in wholesale price developments as well as patterns in generic uptake of simvastatin between the UK and Australia [9]. In Australia the price of statins decreased by 15% annually after patent expiry, down to 50% of the originator price in the first 4 years after patent expiry. In the UK the original price was initially higher, but the price decreased at more than twice the rate to about 4% of the original price over the same period [9]. Additionally Kanavos et al. indicated that both originator and generic prices varied greatly between countries and products and that the minimum generic price is on average 47% lower in countries that use reference pricing for generics [19].
4 Discussion
This systematic review disclosed the evidence scarcity with regard to the impact of patent expiry on drug prices. All included studies suggest that generic entry causes significant price competition that leads to an overall decrease in pharmaceutical costs, though the extent to which drug prices decrease after patent expiry differed between studies and countries. Different trends were observed in price developments after patent expiry between originators and generics. Generic drug prices are negatively correlated with the number of generic manufacturers in the market, although originator prices may increase when more generic manufacturers appear to compensate for losses in market share.
Availability of drug utilization data is important to calculate the overall budget impact, as prices of different products can be weighted by their proportionate use. The majority of the studies (13 out of 16) included drug-utilization [6, 8,9,10, 12, 13, 15, 18, 19, 21,22,23,24]. By correcting overall prices for generic share, strengths, package sizes, and administration forms, the calculated weighted average price represents the price paid by society for a certain drug over time. Of the studies included into this review, five were able to provide an accurate estimate of the price development after patent expiration for the total market by correcting for most these factors [6, 12, 13, 15, 18]. Drug-utilization should preferably be measured in number of DDDs, as this is a global standardized measure that enables price-comparison between different strengths and administration forms.
In order to make useful predictions on future drug price developments or implement data on price developments into cost-effectiveness analyses, the data should be complete, up to date, and specified towards the specific market within the country of interest. Currently available literature shows limitations to do so, especially for European countries. Price developments over the lifetime of drugs may vary greatly between countries as they may apply different pricing and reimbursement policies that can influence drug prices over time [25]. A well-established methodological framework for evaluating drug prices over time that also allows for comparison between countries is lacking. Cross-country price comparisons are only meaningful if comparable data is used to compare the same drug. As such a standardized administration form would be required, as well as an identical format of price display (such as cost per universal outcome measures, e.g., price per defined daily dose). For these reasons the different studies are hardly comparable in terms of price developments over time.
Another limitation is the use of publicly available prices. These prices do not reflect the confidential discounts that are often disclosed between manufacturers and governments or decision makers. Although these prices do not properly reflect on the total healthcare expenditure or possible savings made, these prices are the prices that insurers or consumers have to pay. Consequently, these publicly available list prices are the prices used within assessments of affordability and cost effectiveness studies.
Despite the limitations, data on drug price developments can be utilized in several ways. The horizon scan is a newly introduced method in the Netherlands to track all the innovative drugs that will come to the market as well as drugs that will have their patents expire in the near future [26]. Use of correct information on price developments after patent expiry can help to estimate the impact on the healthcare budget of both these new innovative drugs and those that will have their patent expired. Besides forecasts on healthcare expenditure price developments during the lifecycle of a drug can be used in health economic models to provide more realistic estimations on the cost-effectiveness of a new drug.
The majority of the included studies that focused on the impact of patent expiry on price used data from before 2000, while no study available included data from 2010 onwards. Furthermore, the USA is overly represented in the included studies with only three studies focusing on a single other country. Overall it can be concluded that studies focusing on the impact of patent expiry on the total pharmaceutical and healthcare costs in the European market are still lacking. None of the included studies investigated the impact of patent expiry on price in different therapeutic classes. Stratifying outcomes for therapeutic classes could be interesting as one (e.g., cardiovascular drugs) might be more appealing for generic competition then others. If different therapeutic classes show different trends, then stratification should be necessary in order to make more reliable estimations for price developments of future drugs. Next to therapeutic classes biologicals and orphan drugs should also be placed into a different segment as different regulations apply to these classes of drugs. Moreover, it is important that all drugs that faced generic entry over time are included to provide a weighted estimated price development that is representative for the entire drug market in the country and specific field of interest.
5 Conclusion
With limited evidence and knowledge on the impact of patent expiry on the total pharmaceutical and healthcare costs in the European market, a significant decrease in drug prices after patent expiry was found. The extent of this price reduction varied greatly between products and countries. For this reason, country-specific analyses on price developments after patent expiry should be used when these are considered in decision making. Future research should be dedicated to gathering more country-specific data to reduce the uncertainty on price developments.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
World Bank Group. Health expenditure, total (% of GDP)|Data. https://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Accessed 14 Sept 2017.
Vogler S, Zimmermann N, Leopold C, de Joncheere K. Pharmaceutical policies in European countries in response to the global financial crisis. South Med Rev. 2011;4(2):69–79. https://doi.org/10.5655/smr.v4i2.1004.
Health at a Glance 2015. OECD Publishing; 2015. https://doi.org/10.1787/health_glance-2015-en.
Cameron A, Mantel-Teeuwisse AK, Leufkens HGM, Laing RO. Switching from originator brand medicines to generic equivalents in selected developing countries: how much could be saved? Value Health. 2012;15(5):664–73. https://doi.org/10.1016/j.jval.2012.04.004.
Liberman JN, Roebuck MC. Prescription drug costs and the generic dispensing ratio. J Manag Care Pharm. 2010;16(7):502–6. https://doi.org/10.18553/jmcp.2010.16.7.502.
Lakdawalla DN, Philipson T, Wang R. Intellectual property and marketing. 2007. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=1089045&rec=1&srcabs=328692&alg=7&pos=10. Accessed 14 Sept 2017.
Handoo S, Arora V, Khera D, Nandi PK, Sahu SK. A comprehensive study on regulatory requirements for development and filing of generic drugs globally. Int J Pharm Investig. 2012;2(3):99–105. https://doi.org/10.4103/2230-973X.104392.
Kelton CML, Chang LV, Guo JJ, et al. Firm- and drug-specific patterns of generic drug payments by US medicaid programs: 1991–2008. Appl Health Econ Health Policy. 2014;12(2):165–77. https://doi.org/10.1007/s40258-014-0083-z.
Clarke PM, Fitzgerald EM. Expiry of patent protection on statins: effects on pharmaceutical expenditure in Australia. Med J Aust. 2010;192(11):633–636. http://www.ncbi.nlm.nih.gov/pubmed/20528715. Accessed 14 Sept 2017.
Berndt ER, Mortimer R, Bhattacharjya A, Parece A, Tuttle E. Authorized generic drugs, price competition and consumers’ welfare. Health Aff. 2007;26(3):790–9. https://doi.org/10.1377/hlthaff.26.3.790.
Hong SH, Shepherd MD, Scoones D, Wan TTH. Product-line extensions and pricing strategies of brand-name drugs facing patent expiration. J Manag Care Pharm. 2005;11(9):746–54. https://doi.org/10.18553/jmcp.2005.11.9.746.
Boersma C, Klok RM, Bos JM, et al. Drug costs developments after patent expiry of enalapril, fluoxetine and ranitidine: a study conducted for the Netherlands. Appl Health Econ Health Policy. 2005;4(3):191–196. http://www.ncbi.nlm.nih.gov/pubmed/16309337. Accessed 14 Sept 2017.
Magazzini L, Pammolli F, Riccaboni M. Dynamic competition in pharmaceuticals. Eur J Heal Econ Former HEPAC. 2004;5(2):175–82. https://doi.org/10.1007/s10198-003-0218-x.
Lexchin J. The effect of generic competition on the price of brand-name drugs. Health Policy (New York). 2004;68(1):47–54. https://doi.org/10.1016/j.healthpol.2003.07.007.
Suh DC, Manning WG, Schondelmeyer S, Hadsall RS, Hadsall RS. Effect of multiple-source entry on price competition after patent expiration in the pharmaceutical industry. Health Serv Res. 2000;35(2):529–547. http://www.ncbi.nlm.nih.gov/pubmed/10857475. Accessed 14 Sept 2017.
Grabowski H, Long G, Mortimer R. Recent trends in brand-name and generic drug competition. J Med Econ. 2014;17(3):207–14. https://doi.org/10.3111/13696998.2013.873723.
Grabowski H, Long G, Mortimer R, Boyo A. Updated trends in US brand-name and generic drug competition. J Med Econ. 2016;19(9):836–44. https://doi.org/10.1080/13696998.2016.1176578.
Berndt ER, Aitken ML. Brand loyalty, generic entry and price competition in pharmaceuticals in the quarter century after the 1984 Waxman-Hatch legislation. Int J Econ Bus. 2011;18(2):177–201. http://www.tandfonline.com/loi/cijb20.
Kanavos P, Costa-Font J, Seeley E. Competition in off-patent drug markets: issues, regulation and evidence. Econ Policy. 2008;23(55):499. http://onlinelibrary.wiley.com/journal/10.1111/%28ISSN%291468-0327/issues.
Puig-Junoy J. Do higher-priced generic medicines enjoy a competitive advantage under reference pricing? Appl Health Econ Health Policy. 2012;10(6):441–51. https://doi.org/10.2165/11633810-000000000-00000.
Saha A, Grabowski H, Birnbaum H, Greenberg P, Bizan O. Generic competition in the US pharmaceutical industry. Int J Econ Bus. 2006;13(1):15–38. https://doi.org/10.1080/13571510500519905.
Wiggins SN, Maness R. Price competition in pharmaceuticals: The case of anti-infectives. Econ Inq. 2004;42(2):247–63. https://doi.org/10.1093/ei/cbh058.
Vandoros S, Kanavos P. The generics paradox revisited: empirical evidence from regulated markets. Appl Econ. 2013;45(22):3230–9. https://doi.org/10.1080/00036846.2012.703313.
Grabowski HG, Ridley DB, Schulman KA. Entry and competition in generic biologics. Manag Decis Econ. 2007;28(4–5):439–51. https://doi.org/10.1002/mde.1352.
Zorinstituut Nederland. Geneesmiddelen Horizonscan. https://www.horizonscangeneesmiddelen.nl/binaries/content/assets/horizonscan/gepublicatie-scan-1-september-2017-correctie.pdf. Published 2017. Accessed 15 Sept 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No funding was received for this study.
Author contributions
GTV, MHR, and MJP developed the design of this study. GTV and QC carried out the data collection and conducted the data extraction and were supplemented by MHR for discussion in care of disagreement. All authors contributed in the conceptualization of the paper. GTV and MHR drafted the manuscript. All authors revised it critically for intellectual content. All authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
Mark Rozenbaum is employed by Pfizer. Gert Vondeling, Qi Coa, and Maarten Postma declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vondeling, G.T., Cao, Q., Postma, M.J. et al. The Impact of Patent Expiry on Drug Prices: A Systematic Literature Review. Appl Health Econ Health Policy 16, 653–660 (2018). https://doi.org/10.1007/s40258-018-0406-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-018-0406-6