Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.5844
Peer-review started: August 7, 2020
First decision: September 24, 2020
Revised: September 28, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: December 6, 2020
In the last decades, more efforts are focused on the prevention and treatment of malignant diseases, given the increase in all cancers incidence A lifestyle change, including healthy eating habits and regular physical activity, has significantly impacted colorectal cancer prevention. The effect of dose-dependent physical activity on mortality and recurrence rates of colorectal carcinoma has been unequivocally demonstrated in observational studies. However, clear recommendations are not available on the frequency, duration, and intensity of exercise in patients with colorectal cancer due to the lack of evidence in randomized clinical trials. Regarding pathophysiological mechanisms, the most plausible explanation appears to be the influence of physical activity on reducing chronic inflammation and insulin resistance with a consequent positive effect on insulin growth factor 1 signaling pathways.
Core Tip: Sedentary lifestyle is linked to the development of colorectal cancer (CRC); each 2-h increase in sitting time increases the risk of CRC development by 8%. Additionally, engaging in physical activity can prevent 15% of CRC, and by replacing 30 min of sedentary time with physical activity (either light or moderate-vigorous), cancer mortality can be reduced by 8% and 31%, respectively. Although the mechanisms of preventive and favorable effects of physical activity on CRC are still unknown, the most plausible are the influence on chronic inflammation and insulin resistance, with a positive effect on insulin growth factor 1 signaling pathways.
- Citation: Cigrovski Berkovic M, Cigrovski V, Bilic-Curcic I, Mrzljak A. What is the gut feeling telling us about physical activity in colorectal carcinogenesis? World J Clin Cases 2020; 8(23): 5844-5851
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/5844.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.5844
Colorectal cancer (CRC) is the fourth most common cancer worldwide, with increasing incidence due to lifestyle, environmental changes, and aging demographics; it is estimated to rise to 2.2 million new cases and 1.1 million deaths by 2030[1,2]. Forty-four percent of new CRC diagnoses are older patients, and 20%-25% of CRC is diagnosed at the advanced stage[3]. Five-year overall survival for CRC ranges from 58.4% to 65%[4,5]. CRC screening programs have led to a 16% decline in overall mortality rate without affecting incidence[6]. Thus, strategies that could prevent or decrease the burden of CRC are of major importance.
Sedentary behavior (both viewed through the prism of reduced physical activity and/or time spent on sitting) is emerging as an independent risk factor for chronic diseases such as diabetes, hypertension, obesity, and cardiovascular disease and can directly increase mortality. According to objectively assessed measurements, adults spend 50% to 60% of the day being physically inactive, in sedentary behaviors[7].
Cancer is a leading cause of death in many modern societies, although largely preventable through healthy lifestyle choices[8]. A meta-analysis of 43 observational studies recently linked the sedentary lifestyle to the development of three specific types of cancers; lung, endometrial, and colon. For the later, authors reported an 8% increase of risk in case of each 2-h increase in sitting time[9]. Another meta-analysis, which included 17 prospective studies, reported the associations between sedentary behavior and some cancer types, including CRC [relative risk, 1.30; 95% confidence interval (CI), 1.12-1.49][10].
The Reasons for Geographic and Racial Differences in Stroke study, which included a cohort of 8002 adults aged ≥ 45 years in the United States and prospectively followed them by accelerometry for a mean of 5.7 years, reported an association between greater sedentary time with greater risk of cancer mortality. Moreover, the authors found that replacing 30 min of sedentary time with physical activity (either light or moderate-vigorous) can reduce the cancer mortality for 8% and 31%, respectively[11]. Analysis of cancer risk in relation to the level of leisure-time physical activity involving more than 1.44 million adults showed that a high level of activity provides protection against 13 cancers. For the cancer protection effects, the hazard rates (HR) of cancers in gastrointestinal tracts, namely esophagus, gastric cardia, colon and rectum, are shown to be reduced to 0.58, 0.78, 0.84 and 0.87 respectively with corresponding 95%CI values being 0.37-0.89, 0.64-0.95, 0.77-0.91 and 0.80-0.95. Other cancer protection by elevated physical activities pertains to liver, lungs, kidney, endometrium, breast, bladder, head and neck, and two hematological malignancies (myeloma and myeloid leukemia), with HR in a range of 0.73-0.90. It is noteworthy that these protection effects are irrespective of patients’ body mass index or previous physical fitness. Overall, the higher the physical activity level, the lower the total cancer risk[12]. Similarly, a recently published umbrella review evaluating physical activity and cancer risk showed that regular physical activity might prevent seven types of cancers (colon, breast, endometrium, lung, esophagus, pancreas, and meningioma)[13].
Physical activity offers the protection against colorectal precancerous polyps[14]. There was an exercise duration-related lower prevalence of colon adenomas and polyps in persons who exercised for ≥ 1 h per week as opposed to those who exercised less than an hour weekly[15]. Moreover, the risk for polyp development was decreased throughout the colon, regardless of its specific part. Another study reported the exercise-related decrease in the total number of intestinal polyps, and more specifically in the development of large polyps by 67%[16]. Animal studies suggest preventing of colorectal polyp formation through pro-inflammatory cytokine interleukin-6, which is decreased after treadmill running[17].
Despite the treatment advances, new therapies have a limited impact on cure rates and colon cancer patients’ survival. Therefore, adjuvant treatment strategies, among which lifestyle interventions, are needed. Several observational studies have demonstrated the anti-cancer effects of exercise and its dose-dependent effect on decreasing cancer recurrence mortality and risk. More specifically, physical activity may prevent approximately 15% of colon cancer[18]. A systematic review by Des Guetz and colleagues[19] analyzed six observational studies involving over 7500 CRC survivors and reported that higher post-diagnosis physical activity was associated with a lower risk of CRC-specific mortality (HR, 0.61; 95%CI, 0.44-0.86) and a lower risk of all-cause mortality (HR, 0.62; 95%CI, 0.54-0.71). Despite noticed benefits, no randomized controlled trials have been published exploring the beneficial association between physical activity and survival after CRC treatment. The Challenge Trial[20] is the first prospective randomized controlled trial evaluating the effects (improvements of 3-year disease-free survival, QoL) of structured physical activity on the survival of stage 2 and 3 CRC survivors.
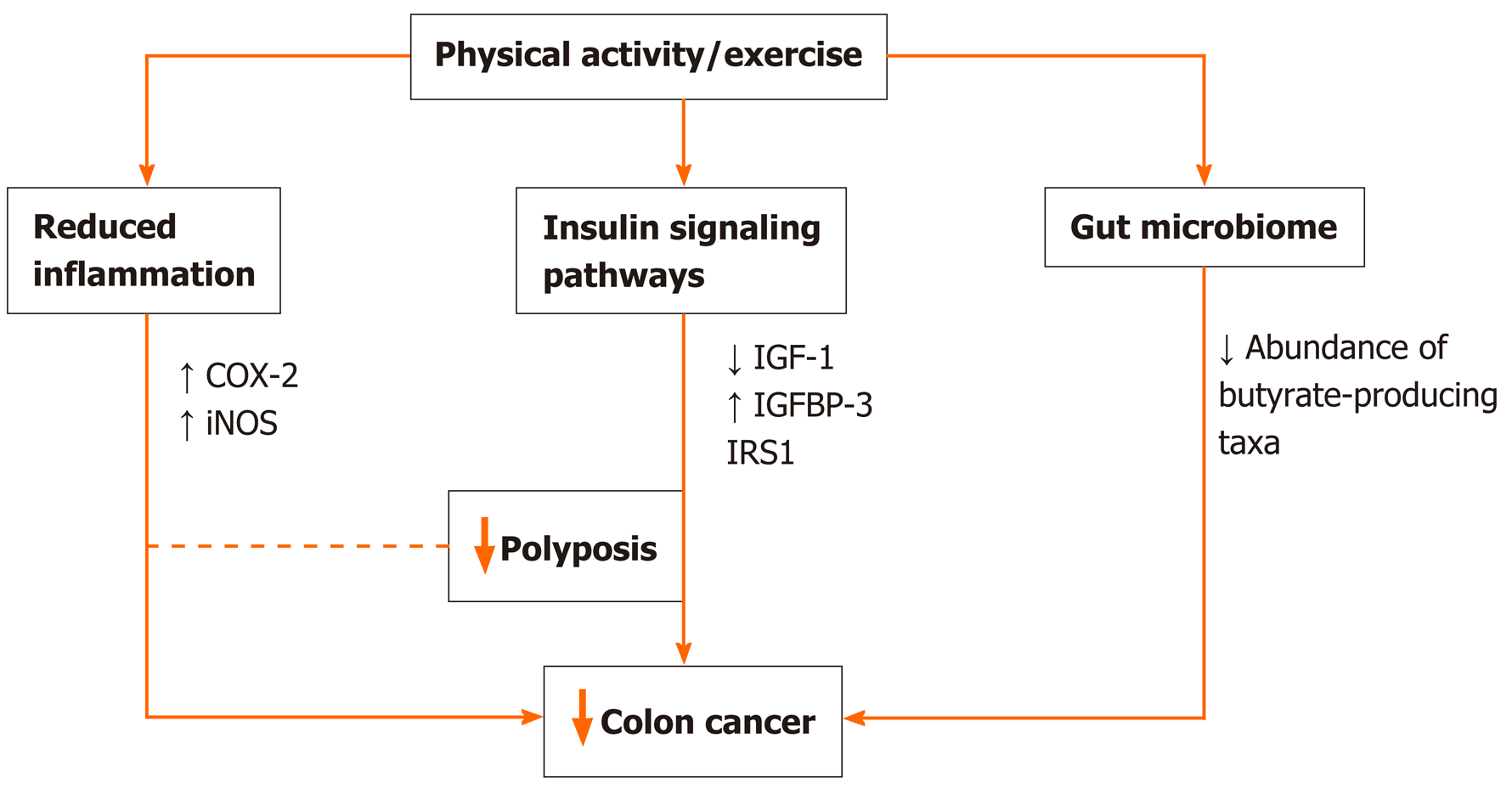
Randomized exercise trials and longitudinal studies initially suggested the link between the preventive role of physical activity on cancer through primarily weight reduction and/or prevention of weight gain[21-23], but nowadays, the association seems more direct and straightforward. There is data suggesting preventive effects even when physical activity lacked the weight loss or maintenance of healthy weight[24J. Potential mechanisms might include a reduction in insulin resistance and inflammation that have been directly linked to colorectal carcinogenesis, or stimulation of digestion and intestinal motility (Figure 1). Moreover, physical activity might directly influence the molecular and genetic pathways involved in colorectal carcinogenesis and improve patient survival[25]. According to available data, physical activity increases the expression of P27 and cyclooxygenase-2 (COX-2) and decreases that of insulin receptor substrate 1 and B-catenin, which correlates to improved cancer-specific survival[26-30]. Another plausible mechanism in need of further exploration is the effect of physical activity on soluble intercellular adhesion molecule, which is known to affect early death and disease recurrence[31].
Inflammation is a well-known risk factor not only for metabolic disorders like obesity, metabolic syndrome, and diabetes but also for CRC. In animal model studies, physical activity decreased systemic inflammation through the effect on pro-inflammatory cytokines and reduced the risk of colonic polyp formation and progression to cancer[32,33]. Additionally, physical activity decreases the inflammation by affecting the expression of inducible nitric oxide synthase and COX-2 in the colon mucosa[34]. COX-2-positive CRC patients engaging in the highest intensity physical activity have a significant decrease in cancer-specific mortality compared to those who are least active[35].
By decreasing insulin resistance and circulating insulin levels, physical activity through its effect on IGF pathway can decrease the risk of initial CRC development and its recurrence, and influence cancer mortality[35,36]. Decreased insulin-like growth factor-1 levels and increased insulin-like growth factor-binding protein 3 levels may be a reasonable mechanism underlying the inverse correlation between CRC and physical activity[37,38]. Study by Lee and coauthors[39] including CRC survivors showed that engagement in postoperative physical activity decreases both insulin levels and insulin resistance, and increases insulin-like growth factor-1 and insulin-like growth factor-binding protein 3 levels. Additionally, insulin receptor substrate 1 involved in the development of insulin resistance can be used as a marker to identify patients who will benefit most from exercise in terms of cancer-related survival[30].
A contracting muscle, via different myokines, enhances insulin sensitivity and, at the same time, decreases the production of pro-inflammatory cytokines, which is a potential mechanism of anti-cancer effect. Recently published studies have shown that specific myokine, named the secretory protein that is acidic and rich in cysteine (SPARC protein) involved in intercellular interaction and cell differentiation, might be important for preventing CRC development by increasing tumor cell apoptosis[39-41].
Changes in the intestinal microbiota composition disrupt gut integrity and induce a systemic inflammatory response, contributing to establishing a chronic low-grade inflammation state with repercussions in the gut and beyond[42]. Therefore targeting microbiota seems like a promising prevention and/or treatment concept. Emerging evidence from animal[43-45] and human studies[46-48] demonstrate that the exercise influences the diversity, composition, and functionality of gut microbiota. In this regard, the exercise increases butyrate-producing taxa and fecal butyrate concentrations and reduces pro-inflammatory cytokines and oxidative stress in the gut[42,46]. There are several potential mechanisms by which physical activity might alter the gut microbiota: Altering the gene expression of lymphocyte (down-regulating pro-inflammatory and up-regulating anti-inflammatory cytokines), alerting temperature, intestinal blood flow, gut motility, the activity of the enteric nervous system, enterohepatic circulation of bile acids or metabolic flux[42]. In the context of CRC, animal studies show that spontaneous wheel activity reduces the incidence of CRC[49].
Furthermore, exercise training reduces tumor multiplicity and the number of large tumors in mice genetically predisposed to intestinal adenomas, substantiating it with a correlation between fecal butyrate concentrations and tumor number[50]. In CRC patients, alternations in gut microbiota are characterized by a reduced abundance of butyrate-producing taxa[51]. Therefore exercise-induced modifications of the intestinal microbiota are likely to have implications in the prevention and treatment of CRC patients.
According to the American Cancer Society guidelines, it is important not only to avoid inactivity but also to be engaged in minimally 150 min cumulative moderate or 75 min vigorous-intensity aerobic exercise weekly, and perform at least two days per week of strength exercises in order to prevent the development of CRC or to have a favorable prognosis[52,53]. The recommendation is to increase the amount of exercise to achieve 300 min of aerobic exercise weekly if possible. Generally, no specific precautions need to be taken if participation in physical activity is incorporated slowly, when time and intensity slowly upgrade. In older individuals or those with known cardiovascular disease, sometimes a medical clearance before engaging in physical activity is needed. Similarly, if patients are on cardiotoxic chemotherapy, a cardiologist’s consult might be prudent[25]. Finally, physical activity improves the patients’ quality of life, functional status, muscle strength, depression, and decreases the risk of CRC recurrence and cancer-specific and overall mortality[54,55].
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Peng X, Sun X S-Editor: Chen XF L-Editor: A P-Editor: Zhang YL
| 1. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Yang T, Li X, Montazeri Z, Little J, Farrington SM, Ioannidis JPA, Dunlop MG, Campbell H, Timofeeva M, Theodoratou E. Gene-environment interactions and colorectal cancer risk: An umbrella review of systematic reviews and meta-analyses of observational studies. Int J Cancer. 2019;145:2315-2329. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24:792-800. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Patel JN, Fong MK, Jagosky M. Colorectal Cancer Biomarkers in the Era of Personalized Medicine. J Pers Med. 2019;9:3. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | National Institutes of Health. Cancer Stat Facts: Colorectal Cancer. 2020. Available from: https://seer.cancer.gov/statfacts/html/colorect.html. [Cited in This Article: ] |
| 6. | Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541-1549. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J. 2011;32:590-597. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097-2116. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Schmid D, Leitzmann MF. Television viewing and time spent sedentary in relation to cancer risk: a meta-analysis. J Natl Cancer Inst. 2014;106:dju098. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Shen D, Mao W, Liu T, Lin Q, Lu X, Wang Q, Lin F, Ekelund U, Wijndaele K. Sedentary behavior and incident cancer: a meta-analysis of prospective studies. PLoS One. 2014;9:e105709. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Gilchrist SC, Howard VJ, Akinyemiju T, Judd SE, Cushman M, Hooker SP, Diaz KM. Association of Sedentary Behavior With Cancer Mortality in Middle-aged and Older US Adults. JAMA Oncol. 2020;6:1210-1217. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, Adami HO, Blair CK, Borch KB, Boyd E, Check DP, Fournier A, Freedman ND, Gunter M, Johannson M, Khaw KT, Linet MS, Orsini N, Park Y, Riboli E, Robien K, Schairer C, Sesso H, Spriggs M, Van Dusen R, Wolk A, Matthews CE, Patel AV. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med. 2016;176:816-825. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Rezende LFM, Sá TH, Markozannes G, Rey-López JP, Lee IM, Tsilidis KK, Ioannidis JPA, Eluf-Neto J. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. 2018;52:826-833. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611-616. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Sanchez NF, Stierman B, Saab S, Mahajan D, Yeung H, Francois F. Physical activity reduces risk for colon polyps in a multiethnic colorectal cancer screening population. BMC Res Notes. 2012;5:312. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Baltgalvis KA, Berger FG, Peña MM, Davis JM, Carson JA. The interaction of a high-fat diet and regular moderate intensity exercise on intestinal polyp development in Apc Min/+ mice. Cancer Prev Res. 2:641-649. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol (1985). 2005;98:2219-2225. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev. 2002;11 Suppl 2:S94-100. [PubMed] [Cited in This Article: ] |
| 19. | Des Guetz G, Uzzan B, Bouillet T, Nicolas P, Chouahnia K, Zelek L, Morere JF. Impact of physical activity on cancer-specific and overall survival of patients with colorectal cancer. Gastroenterol Res Pract. 2013;2013:340851. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Courneya KS, Vardy JL, O'Callaghan CJ, Friedenreich CM, Campbell KL, Prapavessis H, Crawford JJ, O'Brien P, Dhillon HM, Jonker DJ, Chua NS, Lupichuk S, Sanatani MS, Gill S, Meyer RM, Begbie S, Bonaventura T, Burge ME, Turner J, Tu D, Booth CM. Effects of a Structured Exercise Program on Physical Activity and Fitness in Colon Cancer Survivors: One Year Feasibility Results from the CHALLENGE Trial. Cancer Epidemiol Biomarkers Prev. 2016;25:969-977. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Lee IM, Djoussé L, Sesso HD, Wang L, Buring JE. Physical activity and weight gain prevention. JAMA. 2010;303:1173-1179. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569-578. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Ennour-Idrissi K, Maunsell E, Diorio C. Effect of physical activity on sex hormones in women: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. 2015;17:139. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Kuiper JG, Phipps AI, Neuhouser ML, Chlebowski RT, Thomson CA, Irwin ML, Lane DS, Wactawski-Wende J, Hou L, Jackson RD, Kampman E, Newcomb PA. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control. 2012;23:1939-1948. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Oruç Z, Kaplan MA. Effect of exercise on colorectal cancer prevention and treatment. World J Gastrointest Oncol. 2019;11:348-366. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical Activity and Cancer Outcomes: A Precision Medicine Approach. Clin Cancer Res. 2016;22:4766-4775. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Meyerhardt JA, Ogino S, Kirkner GJ, Chan AT, Wolpin B, Ng K, Nosho K, Shima K, Giovannucci EL, Loda M, Fuchs CS. Interaction of molecular markers and physical activity on mortality in patients with colon cancer. Clin Cancer Res. 2009;15:5931-5936. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Morikawa T, Kuchiba A, Yamauchi M, Meyerhardt JA, Shima K, Nosho K, Chan AT, Giovannucci E, Fuchs CS, Ogino S. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA. 2011;305:1685-1694. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Yamauchi M, Lochhead P, Imamura Y, Kuchiba A, Liao X, Qian ZR, Nishihara R, Morikawa T, Shima K, Wu K, Giovannucci E, Meyerhardt JA, Fuchs CS, Chan AT, Ogino S. Physical activity, tumor PTGS2 expression, and survival in patients with colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1142-1152. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Hanyuda A, Kim SA, Martinez-Fernandez A, Qian ZR, Yamauchi M, Nishihara R, Morikawa T, Liao X, Inamura K, Mima K, Cao Y, Zhang X, Wu K, Chan AT, Giovannucci EL, Meyerhardt JA, Fuchs CS, Shivdasani RA, Ogino S. Survival Benefit of Exercise Differs by Tumor IRS1 Expression Status in Colorectal Cancer. Ann Surg Oncol. 2016;23:908-917. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Brown JC, Troxel AB, Ky B, Damjanov N, Zemel BS, Rickels MR, Rhim AD, Rustgi AK, Courneya KS, Schmitz KH. Dose-response Effects of Aerobic Exercise Among Colon Cancer Survivors: A Randomized Phase II Trial. Clin Colorectal Cancer. 2018;17:32-40. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Murphy EA, Enos RT, Velázquez KT. Influence of Exercise on Inflammation in Cancer: Direct Effect or Innocent Bystander? Exerc Sport Sci Rev. 2015;43:134-142. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607-615. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Guffey CR, Fan D, Singh UP, Murphy EA. Linking obesity to colorectal cancer: recent insights into plausible biological mechanisms. Curr Opin Clin Nutr Metab Care. 2013;16:595-600. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, Dobs A, Savage PJ. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147-1154. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV, Karl RC, Coppola D. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30:1128-1133. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Sax AT, Jenkins DG, Devin JL, Hughes GI, Bolam KA, Skinner TL. The insulin-like growth factor axis: A biological mechanism linking physical activity to colorectal cancer survival. Cancer Epidemiol. 2014;38:455-459. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, Pollak MN, Giovannucci EL, Fuchs CS. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27:176-185. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Lee DH, Kim JY, Lee MK, Lee C, Min JH, Jeong DH, Lee JW, Chu SH, Meyerhardt JA, Ligibel J, Jones LW, Kim NK, Jeon JY. Effects of a 12-week home-based exercise program on the level of physical activity, insulin, and cytokines in colorectal cancer survivors: a pilot study. Support Care Cancer. 2013;21:2537-2545. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. 2011;25:811-816. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, Sakuma K, Hang LP, Mizushima K, Hirai Y, Koyama R, Wada S, Higashi A, Kokura S, Ichikawa H, Yoshikawa T. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62:882-889. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | White AK, Smith RJ, Bigler CR, Brooke WF, Schauer PR. Head and neck manifestations of neurofibromatosis. Laryngoscope. 1986;96:732-737. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Matsumoto M, Inoue R, Tsukahara T, Ushida K, Chiji H, Matsubara N, Hara H. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci Biotechnol Biochem. 2008;72:572-576. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Denou E, Marcinko K, Surette MG, Steinberg GR, Schertzer JD. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am J Physiol Endocrinol Metab. 2016;310:E982-E993. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Carbajo-Pescador S, Porras D, García-Mediavilla MV, Martínez-Flórez S, Juarez-Fernández M, Cuevas MJ, Mauriz JL, González-Gallego J, Nistal E, Sánchez-Campos S. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis Model Mech. 2019;12:dmm039206. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD, Woods JA. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc. 2018;50:747-757. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Cronin O, Barton W, Skuse P, Penney NC, Garcia-Perez I, Murphy EF, Woods T, Nugent H, Fanning A, Melgar S, Falvey EC, Holmes E, Cotter PD, O'Sullivan O, Molloy MG, Shanahan F. A Prospective Metagenomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Microbiome of Sedentary Adults. mSystems. 2018;3:e00044-18. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Munukka E, Ahtiainen JP, Puigbó P, Jalkanen S, Pahkala K, Keskitalo A, Kujala UM, Pietilä S, Hollmén M, Elo L, Huovinen P, D'Auria G, Pekkala S. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front Microbiol. 2018;9:2323. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Andrianopoulos G, Nelson RL, Bombeck CT, Souza G. The influence of physical activity in 1,2 dimethylhydrazine induced colon carcinogenesis in the rat. Anticancer Res. 1987;7:849-852. [PubMed] [Cited in This Article: ] |
| 50. | Basterfield L, Mathers JC. Intestinal tumours, colonic butyrate and sleep in exercised Min mice. Br J Nutr. 2010;104:355-363. [PubMed] [DOI] [Cited in This Article: ] |
| 51. | Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6:320-329. [PubMed] [DOI] [Cited in This Article: ] |
| 52. | Liu L, Shi Y, Li T, Qin Q, Yin J, Pang S, Nie S, Wei S. Leisure time physical activity and cancer risk: evaluation of the WHO's recommendation based on 126 high-quality epidemiological studies. Br J Sports Med. 2016;50:372-378. [PubMed] [DOI] [Cited in This Article: ] |
| 53. | King AC, Whitt-Glover MC, Marquez DX, Buman MP, Napolitano MA, Jakicic J, Fulton JE, Tennant BL; 2018 PHYSICAL ACTIVITY GUIDELINES ADVISORY COMMITTEE*. Physical Activity Promotion: Highlights from the 2018 Physical Activity Guidelines Advisory Committee Systematic Review. Med Sci Sports Exerc. 2019;51:1340-1353. [PubMed] [DOI] [Cited in This Article: ] |