Abstract
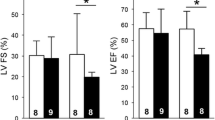
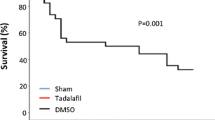
Patients with metabolic syndrome (MetS) often exhibit generalized endothelial and cardiac dysfunction with decreased nitric oxide (NO) production and/or bioavailability. Since phosphodiesterase 5 (PDE5) inhibitors restore NO signaling, we hypothesized that chronic treatment with long-acting PDE5 inhibitor tadalafil may enhance plasma NO levels and reduce cardiac dysfunction following ischemia/reperfusion (I/R) injury in C57BL/6NCrl-Leprdb−lb/Crl mice with MetS phenotypes. Adult male MetS mice were randomized to receive vehicle solvent or tadalafil (1 mg/kg,i.p.) daily for 28 days and C57BL/6NCrl mice served as healthy wild-type controls. After 28 days, cardiac function was assessed by echocardiography and hearts from a subset of mice were isolated and subjected to 30 min of global ischemia followed by 60 min of reperfusion (I/R) in ex vivo Langendorff mode. Body weight, blood lipids, and glucose levels were elevated in MetS mice as compared with wild-type controls. The dyslipidemia in MetS was ameliorated following tadalafil treatment. Although left ventricular (LV) systolic function was minimally altered in the MetS mice, there was a significant diastolic dysfunction as indicated by reduction in the ratio of peak velocity of early to late filling of the mitral inflow, which was significantly improved by tadalafil treatment. Post-ischemic cardiac function, heart rate, and coronary flow decreased significantly in MetS mice compared to wild-type controls, but preserved by tadalafil treatment. Myocardial infarct size was significantly smaller following I/R, which was associated with higher plasma levels of nitrate and nitrite in the tadalafil-treated MetS mice. In conclusion, tadalafil induces significant cardioprotective effects as shown by improvement of LV diastolic function, lipid profile, and reduced infarct size following I/R. Tadalafil treatment enhanced NO production, which may have contributed to the cardioprotective effects.






Similar content being viewed by others
References
Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH (2008) The metabolic syndrome. Endocr Rev 29:777–822. https://doi.org/10.1210/er.2008-0024
Huang PL (2009) A comprehensive definition for metabolic syndrome. Dis Model Mech 2:231–237. https://doi.org/10.1242/dmm.001180
Ford ES (2005) Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 28:1769–1778. https://doi.org/10.2337/diacare.28.7.1769
Hallajzadeh J, Safiri S, Mansournia MA, Khoramdad M, Izadi N, Almasi-Hashiani A, Pakzad R, Ayubi E, Sullman MJ, Karamzad N (2017) Metabolic syndrome and its components among rheumatoid arthritis patients: a comprehensive updated systematic review and meta-analysis. PLoS ONE 12:e0170361. https://doi.org/10.1371/journal.pone.0170361
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT (2002) The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288:2709–2716. https://doi.org/10.1001/jama.288.21.2709
Stocks T, Bjorge T, Ulmer H, Manjer J, Haggstrom C, Nagel G, Engeland A, Johansen D, Hallmans G, Selmer R, Concin H, Tretli S, Jonsson H, Stattin P (2015) Metabolic risk score and cancer risk: pooled analysis of seven cohorts. Int J Epidemiol 44:1353–1363. https://doi.org/10.1093/ije/dyv001
Alkerwi A, Boutsen M, Vaillant M, Barre J, Lair ML, Albert A, Guillaume M, Dramaix M (2009) Alcohol consumption and the prevalence of metabolic syndrome: a meta-analysis of observational studies. Atherosclerosis 204:624–635. https://doi.org/10.1016/j.atherosclerosis.2008.10.036
Bouchard C (1995) Genetics and the metabolic syndrome. Int J Obes Relat Metab Disord 19(Suppl 1):S52–S59
Edwardson CL, Gorely T, Davies MJ, Gray LJ, Khunti K, Wilmot EG, Yates T, Biddle SJ (2012) Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS ONE 7:e34916. https://doi.org/10.1371/journal.pone.0034916
He D, Xi B, Xue J, Huai P, Zhang M, Li J (2014) Association between leisure time physical activity and metabolic syndrome: a meta-analysis of prospective cohort studies. Endocrine 46:231–240. https://doi.org/10.1007/s12020-013-0110-0
Katzmarzyk PT, Leon AS, Wilmore JH, Skinner JS, Rao DC, Rankinen T, Bouchard C (2003) Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc 35:1703–1709. https://doi.org/10.1249/01.MSS.0000089337.73244.9B
Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB (2010) Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 33:2477–2483. https://doi.org/10.2337/dc10-1079
Pollex RL, Hegele RA (2006) Genetic determinants of the metabolic syndrome. Nat Clin Pract Cardiovasc Med 3:482–489. https://doi.org/10.1038/ncpcardio0638
Xi B, He D, Zhang M, Xue J, Zhou D (2014) Short sleep duration predicts risk of metabolic syndrome: a systematic review and meta-analysis. Sleep Med Rev 18:293–297. https://doi.org/10.1016/j.smrv.2013.06.001
Carlstrom M, Larsen FJ, Nystrom T, Hezel M, Borniquel S, Weitzberg E, Lundberg JO (2010) Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci USA 107:17716–17720. https://doi.org/10.1073/pnas.1008872107
Ctoi AF, Parvu AE, Andreicut AD, Mironiuc A, Crciun A, Ctoi C, Pop ID (2018) Metabolically healthy versus unhealthy morbidly obese: chronic inflammation, nitro-oxidative stress, and insulin resistance. Nutrients. https://doi.org/10.3390/nu10091199
Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U (2001) Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 104:342–345. https://doi.org/10.1161/01.cir.104.3.342
Huang PL (2009) eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab 20:295–302. https://doi.org/10.1016/j.tem.2009.03.005
Fernandez ML, Ruiz R, Gonzalez MA, Ramirez-Lorca R, Couto C, Ramos A, Gutierrez-Tous R, Rivera JM, Ruiz A, Real LM, Grilo A (2004) Association of NOS3 gene with metabolic syndrome in hypertensive patients. Thromb Haemost 92:413–418. https://doi.org/10.1160/TH04-02-0103
Monti LD, Barlassina C, Citterio L, Galluccio E, Berzuini C, Setola E, Valsecchi G, Lucotti P, Pozza G, Bernardinelli L, Casari G, Piatti P (2003) Endothelial nitric oxide synthase polymorphisms are associated with type 2 diabetes and the insulin resistance syndrome. Diabetes 52:1270–1275. https://doi.org/10.2337/diabetes.52.5.1270
Bhaswant M, Brown L, McAinch AJ, Mathai ML (2017) Beetroot and sodium nitrate ameliorate cardiometabolic changes in diet-induced obese hypertensive rats. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201700478
Ohtake K, Ehara N, Chiba H, Nakano G, Sonoda K, Ito J, Uchida H, Kobayashi J (2017) Dietary nitrite reverses features of postmenopausal metabolic syndrome induced by high-fat diet and ovariectomy in mice. Am J Physiol Endocrinol Metab 312:E300–E308. https://doi.org/10.1152/ajpendo.00360.2016
Kina-Tanada M, Sakanashi M, Tanimoto A, Kaname T, Matsuzaki T, Noguchi K, Uchida T, Nakasone J, Kozuka C, Ishida M, Kubota H, Taira Y, Totsuka Y, Kina SI, Sunakawa H, Omura J, Satoh K, Shimokawa H, Yanagihara N, Maeda S, Ohya Y, Matsushita M, Masuzaki H, Arasaki A, Tsutsui M (2017) Long-term dietary nitrite and nitrate deficiency causes the metabolic syndrome, endothelial dysfunction and cardiovascular death in mice. Diabetologia 60:1138–1151. https://doi.org/10.1007/s00125-017-4259-6
Sureda A, Bibiloni MD, Martorell M, Buil-Cosiales P, Marti A, Pons A, Tur JA, Martinez-Gonzalez MA, Investigators PS (2016) Mediterranean diets supplemented with virgin olive oil and nuts enhance plasmatic antioxidant capabilities and decrease xanthine oxidase activity in people with metabolic syndrome: the PREDIMED study. Mol Nutr Food Res 60:2654–2664. https://doi.org/10.1002/mnfr.201600450
Behr-Roussel D, Gorny D, Mevel K, Caisey S, Bernabe J, Burgess G, Wayman C, Alexandre L, Giuliano F (2005) Chronic sildenafil improves erectile function and endothelium-dependent cavernosal relaxations in rats: lack of tachyphylaxis. Eur Urol 47:87–91
Schulster ML, Liang SE, Najari BB (2017) Metabolic syndrome and sexual dysfunction. Curr Opin Urol 27:435–440. https://doi.org/10.1097/MOU.0000000000000426
Chaudhary RK, Shamsi BH, Tan T, Chen HM, Xing JP (2016) Study of the relationship between male erectile dysfunction and type 2 diabetes mellitus/metabolic syndrome and its components. J Int Med Res 44:735–741. https://doi.org/10.1177/0300060515623122
Gunduz MI, Gumus BH, Sekuri C (2004) Relationship between metabolic syndrome and erectile dysfunction. Asian J Androl 6:355–358
Kim SC (2000) Hyperlipidemia and erectile dysfunction. Asian J Androl 2:161–166
Vallance P, Chan N (2001) Endothelial function and nitric oxide: clinical relevance. Heart 85:342–350. https://doi.org/10.1136/heart.85.3.342
Sullivan ME, Thompson CS, Dashwood MR, Khan MA, Jeremy JY, Morgan RJ, Mikhailidis DP (1999) Nitric oxide and penile erection: is erectile dysfunction another manifestation of vascular disease? Cardiovasc Res 43:658–665. https://doi.org/10.1016/s0008-6363(99)00135-2
Wheatcroft SB, Williams IL, Shah AM, Kearney MT (2003) Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med 20:255–268
Ockaili R, Salloum F, Hawkins J, Kukreja RC (2002) Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol 283:H1263–H1269
Salloum F, Yin C, Xi L, Kukreja RC (2003) Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 92:595–597
Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, Kukreja RC (2009) Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation 120:S31–S36
Radovits T, Bomicke T, Kokeny G, Arif R, Loganathan S, Kecsan K, Korkmaz S, Barnucz E, Sandner P, Karck M, Szabo G (2009) The phosphodiesterase-5 inhibitor vardenafil improves cardiovascular dysfunction in experimental diabetes mellitus. Br J Pharmacol 156:909–919. https://doi.org/10.1111/j.1476-5381.2008.00098.x
Schafer A, Fraccarollo D, Pfortsch S, Flierl U, Vogt C, Pfrang J, Kobsar A, Renne T, Eigenthaler M, Ertl G, Bauersachs J (2008) Improvement of vascular function by acute and chronic treatment with the PDE-5 inhibitor sildenafil in experimental diabetes mellitus. Br J Pharmacol 153:886–893. https://doi.org/10.1038/sj.bjp.0707459
Aversa A, Greco E, Bruzziches R, Pili M, Rosano G, Spera G (2007) Relationship between chronic tadalafil administration and improvement of endothelial function in men with erectile dysfunction: a pilot study. Int J Impot Res 19:200–207
DeSouza C, Parulkar A, Lumpkin D, Akers D, Fonseca VA (2002) Acute and prolonged effects of sildenafil on brachial artery flow-mediated dilatation in type 2 diabetes. Diabetes Care 25:1336–1339
Deyoung L, Chung E, Kovac JR, Romano W, Brock GB (2012) Daily use of sildenafil improves endothelial function in men with type 2 diabetes. J Androl 33:176–180. https://doi.org/10.2164/jandrol.111.013367
Koka S, Aluri HS, Xi L, Lesnefsky EJ, Kukreja RC (2014) Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1alpha signaling. Am J Physiol Heart Circ Physiol 306:H1558–H1568. https://doi.org/10.1152/ajpheart.00865.2013
Wong SK, Chin KY, Suhaimi FH, Fairus A, Ima-Nirwana S (2016) Animal models of metabolic syndrome: a review. Nutr Metab (Lond) 13:65. https://doi.org/10.1186/s12986-016-0123-9
Ni YG, Condra JH, Orsatti L, Shen X, Di Marco S, Pandit S, Bottomley MJ, Ruggeri L, Cummings RT, Cubbon RM, Santoro JC, Ehrhardt A, Lewis D, Fisher TS, Ha S, Njimoluh L, Wood DD, Hammond HA, Wisniewski D, Volpari C, Noto A, Lo Surdo P, Hubbard B, Carfi A, Sitlani A (2010) A proprotein convertase subtilisin-like/kexin type 9 (PCSK9) C-terminal domain antibody antigen-binding fragment inhibits PCSK9 internalization and restores low density lipoprotein uptake. J Biol Chem 285:12882–12891. https://doi.org/10.1074/jbc.M110.113035
Xi L, Jarrett NC, Hess ML, Kukreja RC (1999) Essential role of inducible nitric oxide synthase in monophosphoryl lipid A-induced late cardioprotection: evidence from pharmacological inhibition and gene knockout mice. Circulation 99:2157–2163
Zhu SG, Kukreja RC, Das A, Chen Q, Lesnefsky EJ, Xi L (2011) Dietary nitrate supplementation protects against Doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol 57:2181–2189. https://doi.org/10.1016/j.jacc.2011.01.024
Frisbee JC (2005) Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 289:R307–R316. https://doi.org/10.1152/ajpregu.00114.2005
Koka S, Das A, Salloum FN, Kukreja RC (2013) Phosphodiesterase-5 inhibitor tadalafil attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in type 2 diabetic mice. Free Radic Biol Med 60:80–88. https://doi.org/10.1016/j.freeradbiomed.2013.01.031
Koka S, Xi L, Kukreja RC (2012) Chronic treatment with long acting phosphodiesterase-5 inhibitor tadalafil alters proteomic changes associated with cytoskeletal rearrangement and redox regulation in Type 2 diabetic hearts. Basic Res Cardiol 107:249. https://doi.org/10.1007/s00395-012-0249-5
Varma A, Das A, Hoke NN, Durrant DE, Salloum FN, Kukreja RC (2012) Anti-inflammatory and cardioprotective effects of tadalafil in diabetic mice. PLoS ONE 7:e45243. https://doi.org/10.1371/journal.pone.0045243
Tada H, Thompson CI, Recchia FA, Loke KE, Ochoa M, Smith CJ, Shesely EG, Kaley G, Hintze TH (2000) Myocardial glucose uptake is regulated by nitric oxide via endothelial nitric oxide synthase in Langendorff mouse heart. Circ Res 86:270–274. https://doi.org/10.1161/01.res.86.3.270
Gwilt DJ, Petri M, Lewis PW, Nattrass M, Pentecost BL (1985) Myocardial infarct size and mortality in diabetic patients. Br Heart J 54:466–472. https://doi.org/10.1136/hrt.54.5.466
Jaffe AS, Spadaro JJ, Schechtman K, Roberts R, Geltman EM, Sobel BE (1984) Increased congestive heart failure after myocardial infarction of modest extent in patients with diabetes mellitus. Am Heart J 108:31–37. https://doi.org/10.1016/0002-8703(84)90541-6
Stone PH, Muller JE, Hartwell T, York BJ, Rutherford JD, Parker CB, Turi ZG, Strauss HW, Willerson JT, Robertson T et al (1989) The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol 14:49–57. https://doi.org/10.1016/0735-1097(89)90053-3
Feuvray D, Lopaschuk GD (1997) Diabetes mellitus and the cardiovascular system. Cardiovasc Res 34:1–2. https://doi.org/10.1016/s0008-6363(97)00071-0
Liu Y, Thornton JD, Cohen MV, Downey JM, Schaffer SW (1993) Streptozotocin-induced non-insulin-dependent diabetes protects the heart from infarction. Circulation 88:1273–1278. https://doi.org/10.1161/01.cir.88.3.1273
Ravingerova T, Neckar J, Kolar F (2003) Ischemic tolerance of rat hearts in acute and chronic phases of experimental diabetes. Mol Cell Biochem 249:167–174
Bonow RO, Udelson JE (1992) Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mech Manag Ann Intern Med 117:502–510. https://doi.org/10.7326/0003-4819-117-6-502
Cregler LL, Georgiou D, Sosa I (1991) Left ventricular diastolic dysfunction in patients with congestive heart failure. J Natl Med Assoc 83:49–52
Zabalgoitia M, Rahman SN, Haley WE, Mercado R, Yunis C, Lucas C, Yarows S, Krause L, Amarena J (1998) Comparison in systemic hypertension of left ventricular mass and geometry with systolic and diastolic function in patients %3c65 to %3e or = 65 years of age. Am J Cardiol 82:604–608. https://doi.org/10.1016/s0002-9149(98)00404-4
Das A, Xi L, Kukreja RC (2005) Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem 280:12944–12955
Franks PW, Luan J, Barroso I, Brage S, Gonzalez Sanchez JL, Ekelund U, Rios MS, Schafer AJ, O'Rahilly S, Wareham NJ (2005) Variation in the eNOS gene modifies the association between total energy expenditure and glucose intolerance. Diabetes 54:2795–2801. https://doi.org/10.2337/diabetes.54.9.2795
Torres SH, De Sanctis JB, Hernandez N, Finol HJ et al (2004) Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J Endocrinol 181:419–427. https://doi.org/10.1677/joe.0.1810419
Acknowledgements
This work was supported by grants from the National Institutes of Health (Grant Nos. CA221813, DK120866, HL118808, HL134366) to R.C.K. S.K. is supported by American Heart Association Institutional Research Enhancement Award (Grant No. 19AIREA34380223) and National Institutes of Health (Grant No. R56HL143809). L.X. is a recipient of Pauley Pilot Research Grant by Virginia Commonwealth University Pauley Heart Center.
Author information
Authors and Affiliations
Contributions
SK and LX performed the experiments and data analysis. SK wrote the manuscript; RCK and LX critically edited the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koka, S., Xi, L. & Kukreja, R.C. Chronic inhibition of phosphodiesterase 5 with tadalafil affords cardioprotection in a mouse model of metabolic syndrome: role of nitric oxide. Mol Cell Biochem 468, 47–58 (2020). https://doi.org/10.1007/s11010-020-03710-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03710-0