- 1Department of Physiotherapy, Bayero University, Kano, Nigeria
- 2Department of Physiotherapy and Rehabilitation Sciences, University of Antwerp, Antwerp, Belgium
- 3Department of Physiotherapy and Rehabilitation, Faculty of Health Sciences, Ordu University, Ordu, Turkey
- 4Department of Physiotherapy, University of Ibadan, Ibadan, Nigeria
- 5Department of Medical Rehabilitation, University of Maiduguri, Maiduguri, Nigeria
- 6Physiotherapy Department, Faculty of Applied Medical Sciences, Umm Al-Qura University, Mecca, Saudi Arabia
- 7NHMRC Center of Clinical Research Excellence in Spinal Pain, Injury and Health, School of Health and Rehabilitation Sciences, University of Queensland, Brisbane, QLD, Australia
- 8Department of Medical Rehabilitation, Nnamdi Azikiwe University, Awka, Nigeria
- 9Muhammad Abdullahi Wase Teaching Hospital, Kano, Nigeria
- 10University of Nottingham, Academic Rheumatology, Nottingham, United Kingdom
Importance: Some of the symptoms of COVID-19 are fever, cough, and breathing difficulty. However, the mechanism of the disease, including some of the symptoms such as the neurological and musculoskeletal symptoms, is still poorly understood.
Objective: The aim of this review is to summarize the evidence on the neurological and musculoskeletal symptoms of the disease. This may help with early diagnosis, prevention of disease spread, and treatment planning.
Data Sources: MEDLINE, EMBASE, Web of Science, and Google Scholar (first 100 hits) were searched until April 17, 2020. The key search terms used were “coronavirus” and “signs and symptoms.” Only studies written in English were included.
Study Selection: The selection was performed by two independent reviewers using EndNote and Rayyan software. Any disagreement was resolved by consensus or by a third reviewer.
Data Extraction and Synthesis: PRISMA guidelines were followed for abstracting data and assessing the quality of the studies. These were carried out by two and three independent reviewers, respectively. Any disagreement was resolved by consensus or by a third reviewer. The data were analyzed using qualitative synthesis and pooled using a random-effect model. Main Outcome(s) and Measure(s): The outcomes in the study include country, study design, participant details (sex, age, sample size), and neurological and musculoskeletal features.
Result: Sixty studies (n = 11, 069) were included in the review, and 51 studies were used in the meta-analysis. The median or mean age ranged from 24 to 95 years. The prevalence of neurological and musculoskeletal manifestations was 35% for smell impairment (95% CI 0–94%; I2 99.63%), 33% for taste impairment (95% CI 0–91%; I2 99.58%), 19% for myalgia (95% CI 16–23; I2 95%), 12% for headache (95% CI 9–15; I2 93.12%), 10% for back pain (95% CI 1–23%; I2 80.20%), 10% for dizziness (95% CI 3–19%; I2 86.74%), 3% for acute cerebrovascular disease (95% CI 1–5%; I2 0%), and 2% for impaired consciousness (95% CI 1–2%; I2 0%).
Conclusion and Relevance: Patients with COVID-19 present with neurological and musculoskeletal symptoms. Therefore, clinicians need to be vigilant in the diagnosis and treatment of these patients.
Key Points
Question: What neurological and musculoskeletal symptoms of COVID-19 are reported in the literature, and what is their prevalence?
Findings: In this review, the reported neurological and musculoskeletal symptoms of COVID-19 are headache, dizziness, impaired consciousness, acute cerebrovascular disease, ataxia, seizure, impaired taste sensation, impaired smell sensation, impaired vision, myalgia, back pain, muscle weakness, skeletal muscle injury, arthralgia, and facial muscle pain. Their prevalence ranges from 1 to 35%.
Meaning: Patients with COVID-19 may present with symptoms such as anosmia, seizure, ataxia, and muscle weakness, which are not among the commonly reported symptoms (fever, cough, and breathing difficulty), and these are still not understood. Therefore, recognizing such symptoms may help in early diagnosis and prevention of the disease. Similarly, it will help with planning the treatment of such symptoms and prevention of further complications in the long term.
Introduction
COVID-19 is the disease associated with a novel coronavirus strain (SARS-CoV-2) belonging to the Nidovirales order, a case of which was first reported in 2019 from Wuhan city in China (1). The disease is said to be transmitted through droplets from human saliva, eyes, and nose (2, 3). When humans come into contact with these droplets, the virus can get into the body through the same routes and lodge in the lungs (2). In the lungs, it will bind with the angiotensin-converting enzymes 2 (ACE 2) in the alveolar cells and destroy them (3, 4). The alveolar cells play important roles in human respiration (5), and their damage can impair the process of respiration. Since the functioning of other systems and organs of the body requires normal functioning of the respiratory system, its impairment will, in turn, impair the functions of those systems and organs, leading to a state of disequilibrium. Consequently, symptoms of COVID-19 can be many and may also vary. So far, the most common notable early symptoms of the disease are believed to be cough, headache, and fever (3). However, recently, evidence is emerging on the effect of COVID-19 on the nervous and musculoskeletal systems (6–8).
The effects of COVID-19 on the nervous and musculoskeletal systems may manifest as anosmia, olfactory function impairment, myalgia, muscle weakness, and Guillian Barre Syndrome (6–9). However, there is still little evidence on these, as scientists are still struggling to understand the disease process, including the pathogenicity, virual replication, and epidemiology (2, 7). Ironically, in some patients, some of these symptoms may precede the commonest symptoms of COVID-19 (10). In addition, symptoms such as myalgia, muscle weakness, and headache may render the patients unable to carry out activities of daily living (ADL) such as walking. In humans, the ability to carry out ADL is associated with good quality of life (11, 12). Furthermore, symptoms such as muscle weakness can result in complications such as muscle atrophy and contracture in the long term. Therefore, identifying the neurological and musculoskeletal features of the disease would be beneficial and can provide further information with which to understand the diagnosis of COVID-19 and how to manage patients. The aim of this review is to summarize the evidence on the neurological and musculoskeletal symptoms of COVID-19.
Methods
Design and Protocol
This is a systematic review and proportional meta-analysis that was conducted according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (13). However, the protocol was not registered in any systematic review register because of the urgent need for literature on COVID-19 that can help curb the spread and impact of the disease.
Eligibility Criteria and Information Sources
The inclusion criteria for this review were as follows: all studies with any study design that reported the neurological and musculoskeletal features in patients with COVID-19, studies published on or prior to April 17, 2020, and studies written in English. However, articles in the form of reviews, anecdotal description, and speculative considerations and editorials were excluded. Studies reporting exclusively on cases in children were also excluded. This is because the majority of the children infected with COVID-19 do not show any symptoms, and even in those who show symptoms, the symptoms tend to be limited to only mild fever and cough (14). Consequently, excluding children can enable us to generalize the findings to the adult population with COVID-19.
Four electronic databases, namely MEDLINE, EMBASE, Web of Science, and Google Scholar (first 100 hits), were searched from their date of establishment to April 17, 2020. The lists of references in the included studies were also screened for any relevant papers. The key search terms used were “coronavirus” and “signs and symptoms,” modified in terms of the glossary of each database and combined using Boolean operators. The search was carried out by one of the reviewers (BK). Appendix 1 demonstrates the search strategy applied in MEDLINE.
Selection of Eligible Studies and Extraction of Data
EndNote and Rayyan were used to remove any duplicates and select eligible studies from the database findings and other sources (lists of references in included studies). Two independent reviewers (AA and SAC) who have experience in conducting systematic reviews selected the eligible studies using Rayyan software (15). Any disagreement between reviewers was resolved by consensus or by a third reviewer (BK).
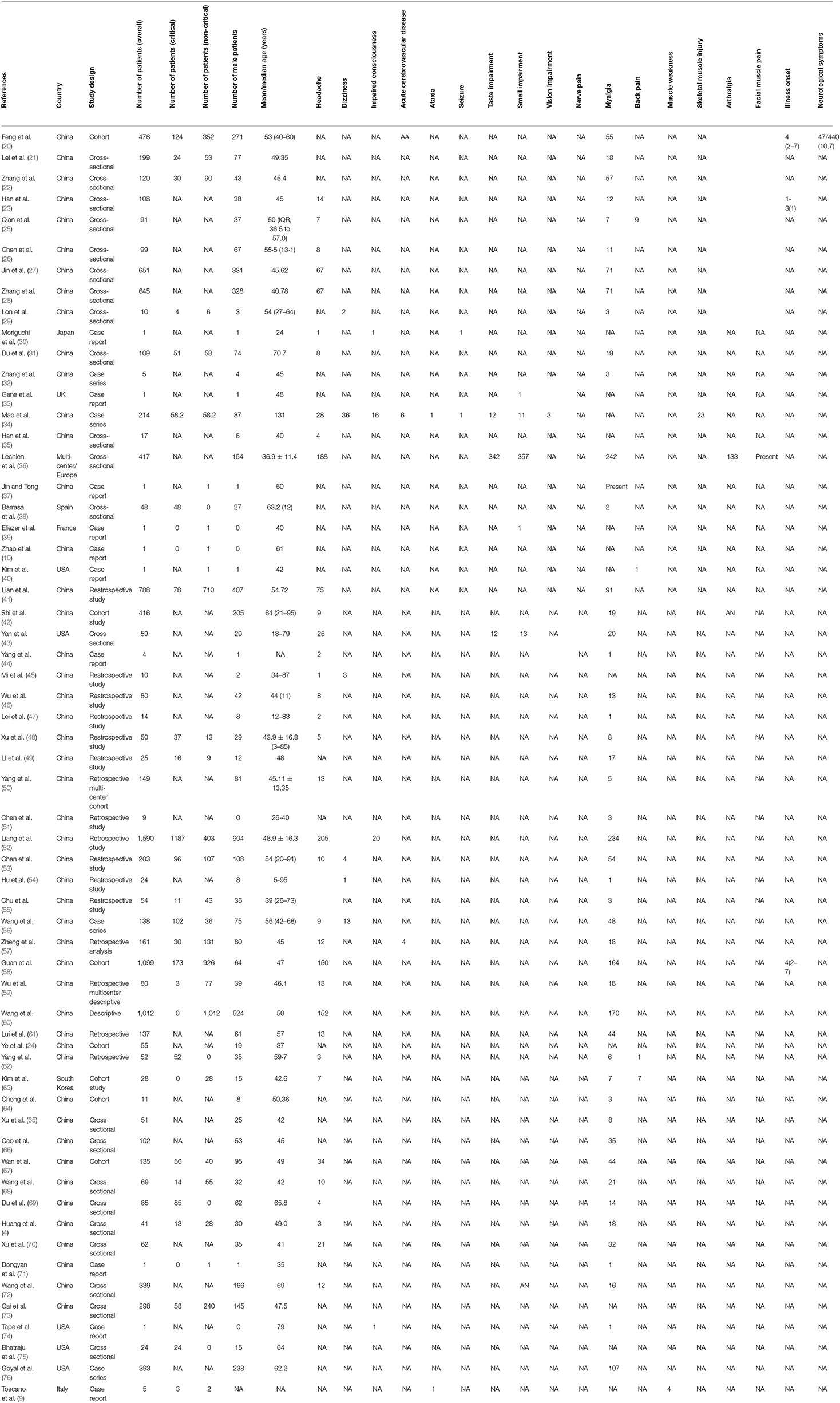
A standardized form was used to extract the relevant data by three reviewers (AA, NUM, and MAA). The data extracted from each study were the study details (study title, first author, year, setting/country, study design), participant details (sex, age, overall sample size, number of patients in critical and non-critical conditions, comorbidities, diagnostic criteria used for the disease (COVID-19), and information about the treatments received), and neurological features, such as headache, dizziness, impaired consciousness, acute cerebrovascular disease, ataxia, seizure, taste impairment, smell impairment, vision impairment, neuropathic pain, and musculoskeletal features such as myalgia and back pain. In addition, the number of patients presenting with a particular symptom was also extracted.
Assessment of the Methodological Quality of the Included Studies
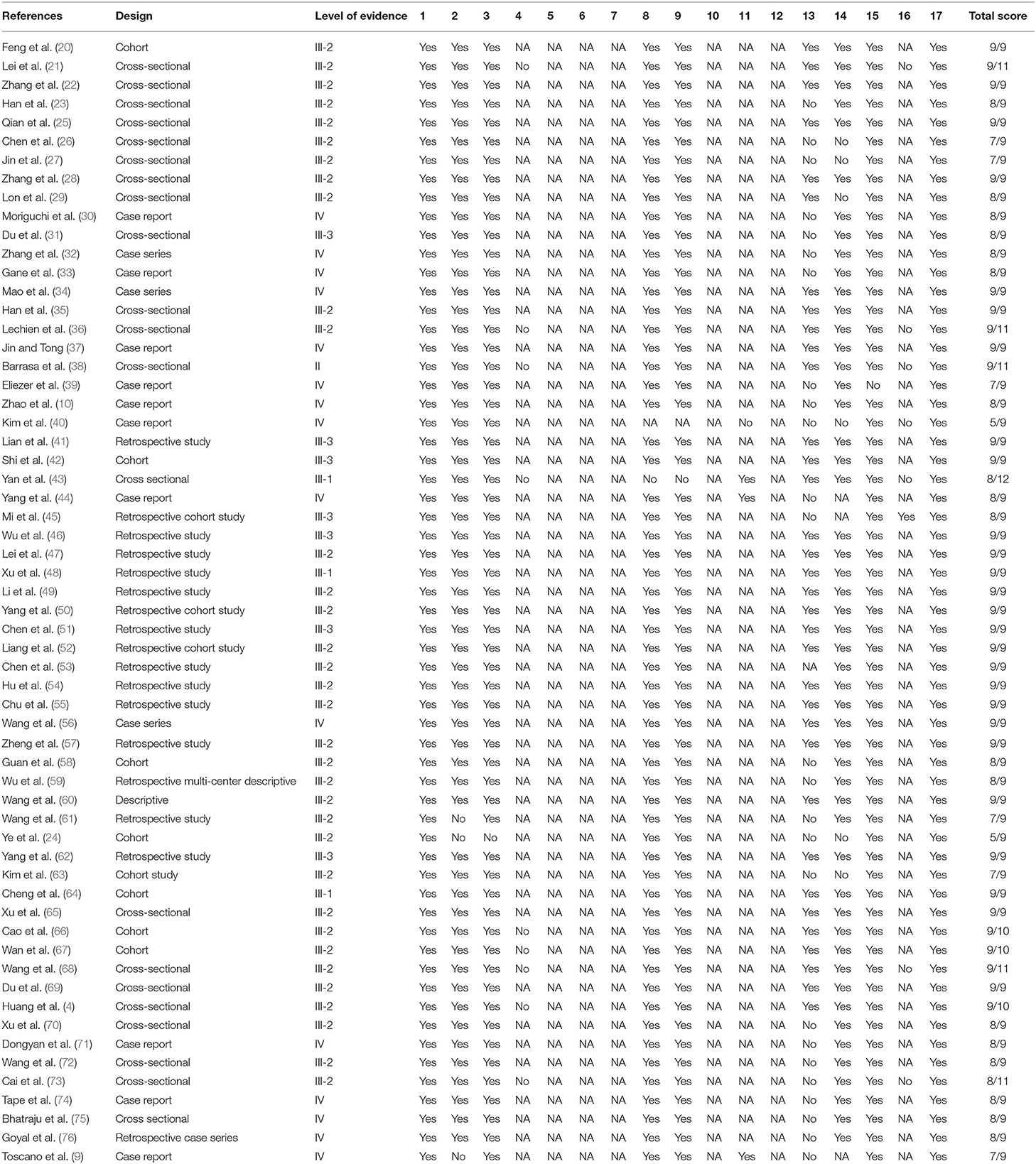
A modified McMaster Critical Review Form for quantitative studies was used to critically assess the methodological quality of the included studies (16). This form is a comprehensive quality tool and can be used to assess all types of quantitative studies. It consists of 17 items with four answer options for each item (yes, no, not addressed and not applicable) to assess seven main components, including study purpose, literature review, study design, sample size, outcomes, interventions, results, and conclusions. Each item receives a score of zero when the answer to a particular item is no or not addressed and a score of one when the answer to a particular item is yes. However, when an item is not applicable to a particular study design, no score is awarded; NA is used to designate this. The scores from the tool are classified as poor, fair, good, or excellent, representing 1/4 or less, ≤2/4, ≥2/4 but ≤3/4, and >3/4 to 4/4 of the total score, respectively. The level of evidence was also determined using the National Health and Medical Research Council's (NHMRC) evidence hierarchy (17). Two independent reviewers performed the quality assessment (ABH and AA). Any disagreements between the first and the second reviewers were resolved through discussion to reach consensus and/or by a third reviewer (NUA). See the McMaster Critical Review Form in Appendix 2.
Data Analysis
Qualitative and quantitative data (descriptive and proportional meta-analysis) analyses were performed. The descriptive analysis was performed and represented in the form of summary tables. The proportional meta-analysis was performed using StataSE 16 for the quantitative data if there were at least two studies that reported the proportion of the same clinical symptom. A random-effect model due to the heterogeneity and Freeman-Tukey double arc-sine transformation were used to stabilize the variance of specific prevalence rates to minimize the impact of studies with extremely small or extremely large prevalence estimates on overall estimates (18). The I2 index was also calculated to assess the level of heterogeneity, which can be classified into four categories: might not be important (0–40%), may represent moderate heterogeneity (30–60%), may represent substantial heterogeneity (50–90%), and considerable heterogeneity (75–100%). Publication bias was assessed using a funnel plot and Egger's test (19).
Results
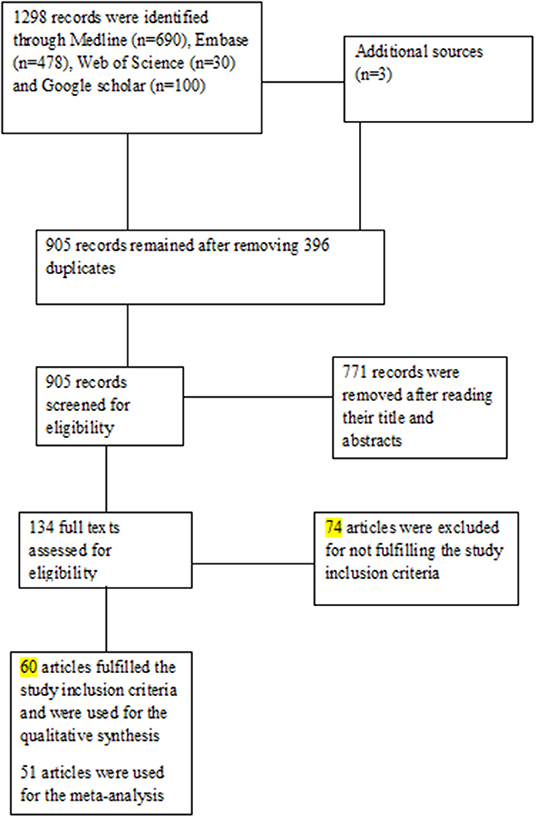
A total of 1,301 published articles were identified from the electronic databases (n = 1,298) and other sources (n = 3). After the removal of duplicate studies (n = 396), 905 studies were eligible for an initial screening based on titles and abstracts. Following the initial screening, 771 records were removed, and the full-texts of 134 articles were screened against the defined eligibility criteria. After the full-text screening, 60 articles (4, 9, 10, 20–76) met the inclusion criteria and were used for qualitative synthesis. For the quantitative synthesis, only 51 articles (4, 9, 20–29, 31, 32, 34–36, 38, 41–43, 45–70, 72, 73, 75, 76) were used. Figure 1 shows the PRISMA flowchart.
The total number of participants in the included studies was 11,069, of which 5,168 were male. The median or mean age of the participants ranges from 24 to 95 years. Based on the studies reporting the situations of the patients, there were 2,377 and 4,882 participants in critical and non-critical conditions, respectively. The most common neurological manifestation was headache (35 studies; 58.33%) (4, 23, 25–28, 30, 31, 34–36, 41–48, 50, 52, 53, 56–63, 67–70, 72), followed by dizziness (6 studies; 10%) (29, 34, 45, 53, 54, 56), impaired smell sensation (5 studies; 8.33%) (33, 34, 36, 43, 55), impaired taste sensation (4 studies; 6.67%) (34, 36, 43, 53), acute cerebrovascular disease (2 studies; 3.33%) (34, 57), ataxia (2 studies; 3.33%) (9, 34), seizure (2 studies; 3.33%) (30, 34), impaired consciousness (1 study; 1.6%) (30), and impaired vision (1 study; 1.6%) (34). However, one study (20) reported non-specified neurological symptoms. The most common musculoskeletal manifestation was myalgia (48 studies; 80%) (4, 20–23, 25–29, 31, 32, 36–38, 41–44, 46–71, 73, 75, 76), followed by back pain (4 studies; 6.67%) (25, 40, 62, 63), muscle weakness (1 study; 1.67%) (9), skeletal muscle injury (1 study; 1.67%) (34), arthralgia (1 study; 1.67%) (36), and facial muscle pain (1 study; 1.67%) (36).
In terms of the study design, four of the studies were case series (22, 34, 56, 76), 10 studies (9, 10, 27, 30, 33, 39, 40, 44, 71, 74) were case reports, and 46 studies (4, 20–26, 28–30, 33, 39, 40, 44, 71, 74) were either cohort or cross-sectional studies. All the studies were published in 2020. The settings/countries of the included studies were as follows: five studies (40, 43, 74–76) were carried out in the United States, and one each was carried out in the United Kingdom (33), Spain (38), Italy (9), South Korea (63), France (39), and Japan (30); one study (36) is a multicenter study carried out in Europe (Belgium, France, Italy, and Spain). The rest of the studies (4, 10, 20–29, 31–35, 37, 41–62, 64–68, 70–73) were carried out in China. In most of the studies, the Chinese national CDC recommended protocol, World Health Organization (WHO) interim guidance, and real-time polymerase chain reaction (RT-PCR) were used to confirm the diagnosis of the disease. There were many comorbidities in the included studies such as hypertension, diabetes, cardiac or cerebrovascular disease, malignancy, chronic kidney disease, pituitary adenoma, chronic obstructive pulmonary disease, chronic renal failure, and cancer. Others are pregnancy, hepatitis B infection, allergic rhinitis, immune-suppression, history of head trauma, and neurological disease. Table 1 shows the details and characteristics of the included studies.
The methodological quality of the included studies was variable. Fifty-eight studies (4, 9, 10, 20–23, 25–39, 41–76) have excellent methodological quality, one study (40) has good methodological quality, and one study (24) has fair methodological quality. In terms of the level of the evidence (based on NHMRC evidence hierarchy), one study is a level II study, three studies are level III-I studies, 35 studies are level III-2 studies, seven studies are level III-3 studies, and 14 studies are level IV studies. Table 2 shows the methodological quality and the level of evidence of the included studies.
The proportional meta-analyses revealed that the prevalence of common neurological and musculoskeletal manifestations was 35% for smell impairment (95% CI 0–94%; I2 99.63%), 33% for taste impairment (95% CI 0–91%; I2 99. 58%), 19% for myalgia (95% CI 16–23; I2 95%), 12% for headache (95% CI 9–15; I2 93.12%), 10% for back pain (95% CI 1–23%; I2 80.20%), 10% for dizziness (95% CI 3–19%; I2 86.74%), 3% for acute cerebrovascular disease (95% CI 1–5%; I2 0%), and 2% for impaired consciousness (95% CI 1–2%; I2 0%). Figure 2 shows the forest plots for the prevalence of acute cerebrovascular disease, impaired consciousness, back pain, dizziness, headache, myalgia, smell impairment, and taste impairment.
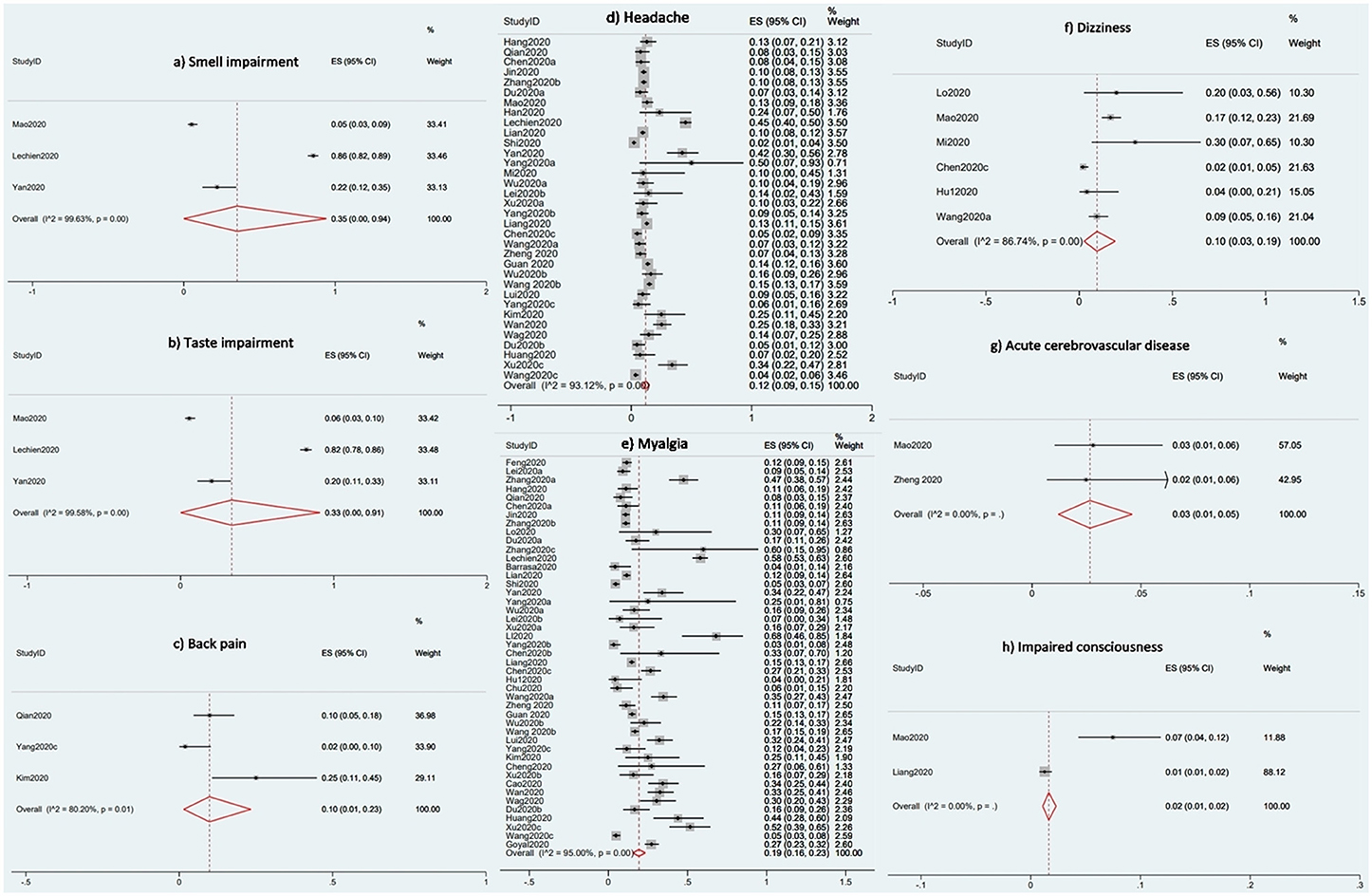
Figure 2. Forest plots for the prevalence of the Neurological and Musculoskeletal Features of Covid-19.
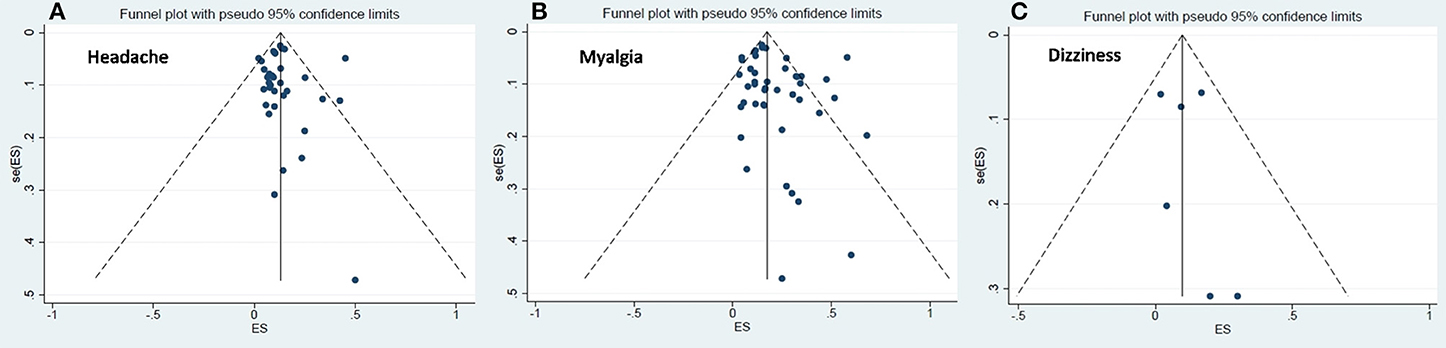
The visual symmetry of the funnel plots suggests that there was no publication bias for headache, dizziness, and myalgia. These results were also confirmed by Egger's test, which revealed statistically insignificant p-values. See Figure 3 for the funnel plots.
Discussion
The results showed that the prevalence of neurological and musculoskeletal manifestations of COVID-19 was 35% for smell impairment, 33% for taste impairment, 19% for myalgia, 12% for headache, 10% for back pain, 3% for acute cerebrovascular disease, and 2% for impaired consciousness. In addition, the majority of the studies have excellent methodological quality, which is an indication of the validity and reliability of the studies (77). Thus, it is important that clinicians consider these symptoms during diagnosis of the disease and management of the patients to help prevent the spread of the disease and the development of any complications. For instance, acute cerebrovascular disease can manifest as symptoms such as stroke, seizure, and headache, which can result in long-term disability that may require rehabilitation for a very long time (78–80). Similarly, symptoms such as muscle weakness, myalgia, vision impairment, and arthralgia can interfere with patients' ability to carry out activities of daily living (ADL). When people are able to carry out ADL, they tend to have better quality of life (11, 12).
In addition, two of the most important factors about COVID-19 are that it is highly contagious and most of the people infected may not present with any notable symptoms such as fever and cough (56). This means that the presence of some previously unnoticed symptoms such as muscle weakness, visual impairment, and arthralgia may not raise any suspicion of the disease. As such, many unnoticed cases could infect many others and increase the spread of the disease. Delineating the whole spectrum of the symptoms patients with COVID-19 present with can help with prompt diagnosis, isolation, and treatment of cases.
One important finding in this study is that there are more neurological symptoms than musculoskeletal symptoms in patients with COVID-19. This may not be surprising, as the virus is believed to be neurotrophic, and the patients may therefore present with neurological symptoms or complications, especially in the long term (81, 82). Similarly, it is also possible that the patients will present with more musculoskeletal symptoms and complications in the long-term due to prolonged immobilization (83, 84). It is also worth noting that many of the symptoms in patients with COVID-19 are non-specific and cannot be highlighted as support for the early diagnosis of the disease. For instance, symptoms such as headache and impaired consciousness may be related to the respiratory failure. However, based on the reviewed studies reporting on the situations of the patients, the majority of the participants were not in critical condition. Therefore, it cannot be said with certainty that these non-specific symptoms are the result of respiratory failure.
Nevertheless, several factors may be the likely causes of the neurological and musculoskeletal features of COVID-19. Firstly, the virus may gain access to, for example, the central nervous system via the bloodstream and infect endothelial cells or leukocytes or through retrograde neuronal routes by infecting the peripheral nerves (85). Secondly, the virus causes pneumonia, which may result in systemic hypoxia, which will eventually damage the brain and other nerve cells (86). The processes through which the damage occurs include peripheral vasodilatation, hypercabia, hypoxia, and anaerobic metabolism, which ultimately result in neuronal swelling and brain edema (87). Neural swelling and brain edema can raise intracranial pressure and result in impaired consciousness and seizure or can irritate the trigeminal nerve and cause headache (88, 89). In addition, cytokine storms characterized by increased levels of inflammatory cytokines and activities of T lymphocytes, macrophages, and endothelial cells can also cause neuronal damage. In particular, the release of interleukin-6 causes vascular leakage and activation of complement and coagulation cascades (90). Consequently, it was noted that patients with the severe disease (COVID-19) tend to have higher levels of D-dimer, which is a marker of a hypercoagulable state and endogenous fibrinolysis (34, 91). These may be the factors that cause acute cerebrovascular disease in patients with COVID-19. Similarly, the elevated level of serum interleukin-6 during cytokine storms could be the cause of myalgia (92). The cytokine storm may also be the cause of the arthralgia presented by the patients. This is because interleukin-6 is a pro-inflammatory substance (93), and viral infections are also known to cause arthralgia (94). Thus, it is possible that arthralgia, which is joint pain, is associated with myalgia in patients with COVID-19.
Although we excluded studies in children because they generally present only with mild fever and cough (14), we recommend that they should be kept under close observation, since damage to the developing nervous system can be devastating. According to the World Health Organization (WHO), recent findings on symptoms in children testing positive for COVID-19 have shown unexplained inflammatory syndrome, mostly in several European and North American countries (95). However, due to the uncertainties of the definitions of symptoms associated with COVID-19 in children, it is important that more evidence is allowed to emerge before their presenting symptoms are categorized into definite neurological and/or musculoskeletal symptoms (95).
This review has multiple strengths, such as the estimation of prevalence for both neurological and musculoskeletal manifestations, the inclusion of a large number of studies (n = 60) with considerable sample size (n = 11, 069), the assessment of methodological quality and the level of evidence, and the use of proportional meta-analyses for the quantitative data. In addition, even though two systematic reviews on the neurological features of COVID-19 have been published previously (96, 97), this review seems to be the only one reporting symptoms such low-back and facial pain. Similarly, the study also has some limitations. One of the limitations is that the reviewed studies could not account for whether or not the neurological and musculoskeletal symptoms of COVID-19 are due to the comorbidities and/or the medicines the patients use for the comorbidities. This is because a number of comorbidities are reported in the studies, and possibly the comorbidities or the drugs the patients take may be responsible for one or more of these neurological or musculoskeletal symptoms. Other limitations are related to the search process, where gray literature databases were not searched and non-English language studies were not included. However, the lists of references of all included studies were screened to include all relevant studies and reduce the risk of publication bias. Furthermore, the heterogeneity between studies was high in most of the meta-analysis results. This may impact negatively on the certainty of the findings.
Conclusions
Patients with COVID-19 present with many different symptoms, including those that affect the neurological and musculoskeletal systems. Therefore, delineating the whole spectrum of symptoms of the disease can help with early diagnosis, prevention of the spread of the disease, and its treatment. In addition, it will help with the prevention of complications that may arise in the long term.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
AA conceived and designed (initially) the study and wrote up the results (with inputs from BK) and the discussion. SC, BK, NU, AB, EA, MAb, and MAl provided inputs to improve the design of the study. In particular, SC and BK modified the search strategy and the data extraction form. BK searched the literature. AA and SC selected the studies for eligibility. AA, NU, and MAb extracted the study data. AB, AA, and NU did the assessment of the methodological quality of the included studies. AA did the qualitative synthesis, and BK did the meta-analysis. AA and EA wrote up the introduction. MAl wrote up the methodology, which was modified by AA. SC, BK, EA, and MAl critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00687/full#supplementary-material
References
1. Xu T, Chen C, Zhu Z, Cui M, Chen C, Dai H, et al. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis. (2020) 64:68–71. doi: 10.1016/j.ijid.2020.03.022
2. Zhiwen Hu, Zhongliang Y, Qi L, Yongfeng H. Infodemiological study on COVID-19 epidemic and COVID-19 infodemic. (2020). doi: 10.21203/rs.3.rs-18591/v1
3. Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. (2020) 92:548–51. doi: 10.1002/jmv.25722
4. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
5. Castranova V, Rabovsky J, Tucker JH, Miles PR. The alveolar type II epithelial cell: a multifunctional pneumocyte. Toxicol Appl Pharmacol. (1988) 93:472–83. doi: 10.1016/0041-008X(88)90051-8
6. Adhikari SP, Meng S, Wul Y-J, Mao Y-P, Ye R-X, Wang Q-Z, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. (2020) 9:29. doi: 10.1186/s40249-020-00646-x
7. Ahmed MU, Hanif M, Ali MJ, Haider MA, Kherani D, Memon GM, et al. Neurological manifestations of COVID-19 (SARS-CoV-2): a review. Front Neurol. (2020) 22:518. doi: 10.3389/fneur.2020.00518
8. Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: a review. Acta Neurol Scand. (2020) 142:14–22. doi: 10.1111/ane.13266
9. Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain-Barre syndrome associated with SARS-CoV-2. N Eng J Med. (2020) 7:2050313X19838750. doi: 10.1056/NEJMc2009191
10. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. (2020) 19:383–4. doi: 10.1016/S1474-4422(20)30109-5
11. Hartman-Maeir A, Soroker N, Ring H, Avni N, Katz N. Activities, participation and satisfaction one-year post stroke. Disabl Rehabil. (2007) 29:559–66. doi: 10.1080/09638280600924996
12. Huang YH, Wu CY, Hsieh YW, Lin KC. Predictors of change in quality of life after distributed constraint-induced therapy in patients with chronic stroke. Neurorehabil Neural Repair. (2010) 24:559–66. doi: 10.1177/1545968309358074
13. Liberati A, Altman DG, Tetzlaff J, Mulrow C. The PRISMA statement for reporting systematic reviews and meta-analyses of studies evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
14. Su L, Ma X, Yu H, Zhang Z, Bian P, Han Y, et al. The different clinical characteristics of Corona Virus Disease cases between children and their families in China - the character of children with COVID-19. Emerg Microbes Infect. (2020) 9:707–13. doi: 10.1080/22221751.2020.1744483
15. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan- a web and mobile application for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
16. Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk WM, et al. Chemokine upregulation in SARS coronavirus infected human monocyte derived dendritic cells. Blood. (2005) 106:2366–76. doi: 10.1182/blood-2004-10-4166
17. Council-NHaMR. NHMRC levels of evidence and grades for recommendations for guideline developers. Canberra, ACT: National Health and Medical Research Council (2009).
18. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Commun Health. (2013) 67:974–8. doi: 10.1136/jech-2013-203104
19. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Book Series. Chichester: Willey-Blackwell Publications (2011).
20. Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with different severity: A multi-center study of clinical features. Am J Respir Crit Care Med. (2020) 201:1380–88. doi: 10.1164/rccm.202002-0445OC
21. Lei Z, Cao H, Yusheng Jie Y, Huang Z, Guo X, Chen J, et al. A cross-sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID-19) in Wuhan and outside Wuhan, China. Travel Med Infect Dis. (2020) 101664. doi: 10.1016/j.tmaid.2020.101664
22. Zhang R, Ouyang H, Fu L, Wang S, Han J, Huang K, et al. CT features of SARS-Cov-2 pneumonia according to clinical presentation: a retrospective analysis of 120 consecutive patients from Wuhan city. Eur Radiol. (2020) 1–10. doi: 10.1007/s00330-020-06854-1
23. Han R, Huang L, Jiang H, Dong J, Peng H, Zhang D. Early clinical and CT manifestations of Coronavirus Disease 2019 (COVID-19) pneumonia. Am J Roentgenology. (2020) 1–6. doi: 10.2214/AJR.20.22961
24. Ye G, Pan Z, Pan Y, Deng Q, Chen L, Li J, et al. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J Infect. (2020) 80:e14–17. doi: 10.1016/j.jinf.2020.03.001
25. Qian GQ, Yang NB, Ding F, Ma AHY, Wang ZY, Shen YF, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: A retrospective, multi-center case series. QJM Int J Med. (2020) hcaa089. doi: 10.1093/qjmed/hcaa089
26. Chen H, Guo J, Wang C, Luo F, Yu X, Wei Z, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. (2020) 395:809–15. doi: 10.1016/S0140-6736(20)30360-3
27. Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. (2020) 69:1002–9. doi: 10.1136/gutjnl-2020-320926
28. Zhang X, Cai H, Hu J, Lian J, Gu J, Zhang S, et al. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int J Infec Dis. (2020) 94:81–87. doi: 10.1016/j.ijid.2020.03.040
29. Lon IL, Lio CF, Cheong HH, Lei CI, Cheong TH, Zhong X, et al. Evolution of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. (2020) 16:1698–707. doi: 10.7150/ijbs.45357
30. Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-CoV-2. Int J Infect Dis. (2020) 94:55–8. doi: 10.1016/j.ijid.2020.03.062
31. Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: A retrospective observational study. Am J Res Crit Care Med. (2020) 201:1372–9. doi: 10.2139/ssrn.3546088
32. Zhang H, Chen Y, Yuan Q, Xia QX, Zeng XP, Peng JT, et al. Identification of Kidney transplant recipients with coronavirus disease (2019). Eur J Urol. (2020) 77:742–7. doi: 10.1016/j.eururo.2020.03.030
33. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. (2020) 58:299–301. doi: 10.4193/Rhin20.114
34. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. J Am Med Assoc. (2020) e201127. doi: 10.1001/jamaneurol.2020.1127
35. Han X, Cao Y, Jiang N, Chen Y, Alwalid O, Zhang X, et al. Novel coronavirus pneumonia (COVID-19) progression course in 17 discharged patients: comparison of clinical and thin-section CT features during recovery. Clin Infect Dis. (2020) ciaa271. doi: 10.1093/cid/ciaa271
36. Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. (2020) 1–11. doi: 10.1007/s00405-020-05965-1
37. Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. (2020) 26. doi: 10.3201/eid2607.200445
38. Barrasa H, Rello J, Tejada S, Martin A, Balziskueta G, Vinuesa C, et al. SARS-CoV-2 in Spanish intensive care: early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. (2020). doi: 10.1016/j.accpm.2020.04.001. [Epub ahead of print].
39. Eliezer M, Hautefort C, Hamel AL, Verillaud B, Herman P, Houdart E, et al. Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. (2020). doi: 10.1001/jamaoto.2020.0832. [Epub ahead of print].
40. Kim J, Thomsen T, Sell N, Goldsmith AJ. Abdominal and testicular pain: An atypical presentation of COVID-19. Am J Emerg Med. (2020). doi: 10.1016/j.ajem.2020.03.052. [Epub ahead of print].
41. Lian J, Jin X, Hao S, Cai H, Zhang S, Zheng L, et al. Analysis of epidemiological and clinical features in older patients with Corona Virus disease 2019 (COVID-2019) out of Wuhan. Clin Infect Dis. (2020) ciaa242. doi: 10.1093/cid/ciaa242
42. Shi S, Qin M, Shen B, Cai Y, Liu Y, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) e200950. doi: 10.1001/jamacardio.2020.0950
43. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int forum Allergy Rhinol. (2020). doi: 10.1002/alr.22579. [Epub ahead of print].
44. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
45. Mi B, Chen L, Xiong Y, Xue H, Zhou W, Liu G, et al. Characteristics and early prognosis of COVID-19 infection in fracture patients. J Bone Joint Surg. (2020) 102:750–8. doi: 10.2106/JBJS.20.00390
46. Wu J, Wu X, Zeng W, Guo D, Fang Z, Chen L, et al. Chest CT findings in patients with Coronavirus disease 2019 and its relationship with clinical features. Invest Radiol. (2020) 55:257–61. doi: 10.1097/RLI.0000000000000670
47. Lei P, Huang Z, Liu G, Wang P, Song W, Mao J, et al. Clinical and computed tomographic (CT) images characteristics in the patients with COVID-19 infection: what should radiologist need to know? J X-ray Sci Tech. (2020) 1–13. doi: 10.3233/XST-200670
48. Xu WH, Dong JH, An WM, Lu XY, Yin XP, Zhang JZ, et al. Clinical and computed tomographic features of novel coronavirus pneumonia caused by SARS-Cov-2. J Infect. (2020) 80:394–400. doi: 10.1016/j.jinf.2020.02.017
49. Li YK, Peng S, Li LQ, Wabg Q, Ping W, Zhang N, et al. Clinical and transmission characteristics of COVID-19 A retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci. (2020) 40:1–6. doi: 10.1007/s11596-020-2176-2
50. Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. (2020) 80:388–93. doi: 10.1016/j.jinf.2020.02.016
51. Chen TL, Dai Z, Mo P, Li X, Ma Z, Song S, et al. Clinical characteristics and outcomes of older patients with Coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single centered, retrospective study. J Garontol Ser A. (2020) glaa089. doi: 10.1093/gerona/glaa089
52. Liang WH, Guan WJ, Li CH, Li YM, Liang HR, Zhao Y, et al. Clinical characteristics and outcomes of hospitalized patients with COVID-19 treated in Hubei (epicenter) and outside Hubei (non-epicenter): a nationwide analysis of China. Eur Respir J. (2020) doi: 10.1183/13993003.00562-2020
53. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 case of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
54. Hu Z, Song C, Xu C, Jin G, Chen Y, Yu X, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci Chi Life Sci. (2020) 63:706–11. doi: 10.1007/s11427-020-1661-4
55. Chu J, Yang N, Wei Y, Yue H, Zhang F, Zhao J, et al. Clinical characteristics of 54 medical staffs with COVID-19: A retrospective study in a single center in Wuhan, China. J Med Virol. (2020) 92:807–13. doi: 10.1002/jmv.25793
56. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus – infected pneumonia in Wuhan – China. J Am Med Assoc. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
57. Zheng F, Tang W, Li H, Huang LX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (Covid-19) in Changsha. Eur Rev Med Pharmacol Sci. (2020) 24:3404–10. doi: 10.26355/eurrev_202003_20711
58. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Eng J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
59. Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. (2020) ciaa199. doi: 10.1093/cid/ciaa199
60. Wang X, Fang J, Zhu Y, Chen I, Ding F, Zhou R, et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang hospital. Clin Microbiol Infect. (2020). doi: 10.1016/j.cmi.2020.03.032. [Epub ahead of print].
61. Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei province. Chin Med J. (2020) 133:1025–31. doi: 10.1097/CM9.0000000000000744
62. Yang Z, Le Y, Ming H, Minqiang H, Xuedong S, Weidong Z, et al. Case report on early diagnosis of COVID-19. Disaster Med Pub Health Preparedness. (2020) 1–4. doi: 10.1017/dmp.2020.66
63. Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. (2020) 35:e142. doi: 10.3346/jkms.2020.35.e142
64. Cheng Z, Lu Y, Cao Q, Qin L, Pan Z, Yan F, et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. Am J Roentgenol. (2020) 1–6. doi: 10.2214/AJR.20.22959
65. Xu XW, Wu XX, Jiang XG, Xu Kl, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: retrospective case series. BMJ. (2020) 368:m792. doi: 10.1136/bmj.m792
66. Cao J, Tu WJ, Cheng W, Yu L, Liu YK, Hu X, et al. Clinical features and short-term outcomes of 102 patients with Corona Virus disease 2019 in Wuhan, China. Clin Infect Dis. (2020) ciaa43. doi: 10.1093/cid/ciaa243
67. Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol. (2020) 92:797–806. doi: 10.1002/jmv.25783
68. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. (2020) ciaa272. doi: 10.1093/cid/ciaa272
69. Du RH, Liu LM, Yin W, Wang W, Guan LL, Yuan ML, et al. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann Am Thoracic Soc. (2020). doi: 10.1513/AnnalsATS.202003-225OC. [Epub ahead of print].
70. Xu J, Li Y, Gan F, Du Y, Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res. (2020). doi: 10.1177/0022034520918518. [Epub ahead of print].
71. Dongyan C, Ai Z, Aiguo L, Qun H. Clinical findings in a patient with hemophilia A affected by COVID-19. Hemophilia. (2020)
72. Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. (2020) 80:639–45. doi: 10.1016/j.jinf.2020.03.019
73. Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID-19 in a designated infectious disease hospital outside Hubei Province, China. Allergy. (2020). doi: 10.1111/all.14309. [Epub ahead of print].
74. Tape C, Byrd KM, Aung S, Lonks JR, Flanigan TP, Rybak NR. COVID-19 in a patient presenting with syncope and a normal chest X-ray. Rhode Island Med J. (2020) 103:50–51.
75. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla Ak. COVID-19 in critically ill patients in the Seattle region – case series. N Eng J Med. (2020) 382:2012–22. doi: 10.1056/NEJMoa2004500
76. Goyal P, Choi JJ, Schenck Chen R, Jabri A, Satlin MJ, et al. Clinical characteristics of COVID-19 in New York city. N Eng J Med. (2020) 382:2372–4. doi: 10.1056/NEJMc2010419
77. Terwee CB, Mokkink LB, Knol DL, Ostelo RW, Bouter LM, de Vet HC. Rating the methodological quality in systematic reviews of studies on measurement properties: A scoring system the COSMIN checklist. Qual LR. (2012) 21:651–7. doi: 10.1007/s11136-011-9960-1
78. Arboix AJ, Massons M, Oliveres MP, Titus AF. Headache in acute cerebrovascular disease: a prospective clinical study in 240 patients. (1994) 14:37–40. doi: 10.1046/j.1468–2982.1994.1401037.x
79. Arboix A, García-Eroles L, Massons JB, Oliveres M, Comes E. Predictive factors of early seizures after acute cerebrovascular disease. Stroke. (1997) 28:1590–4. doi: 10.1161/01.STR.28.8.1590
80. Rolfs A, Fazekas F, Grittner U, Dichgans M, Martus P, Holzhausen M, et al. Acute cerebrovascular disease in the young: the stroke in young fabry patients study. Stroke. (2013) 44:340–9. doi: 10.1161/STROKEAHA.112.663708
81. Baig AM, Khaleeq A, Ali U, Hira Syeda H. Evidence of the COVID-19 virus targeting the NS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. (2020) 11:995–8. doi: 10.1021/acschemneuro.0c00122
82. Pereira A. Long-term neurological threats of COVID-19: a call to update the thinking about the outcomes of the coronavirus pandemic. Front Neurol. (2020) 1:308. doi: 10.3389/fneur.2020.00308
83. Parry SM, El-Ansary D, Cartwright MS, Sarwal A, Berney S, Koopman R, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. (2015) 30:1151.e9-14. doi: 10.1016/j.jcrc.2015.05.024
84. Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. (2013) 310:1591–600. doi: 10.1001/jama.2013.278481
85. Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dube M, et al. Human Coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. (2019) 12:14. doi: 10.3390/v12010014
86. Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. (2020) doi: 10.1016/j.jocn.2020.05.017
87. Tu H, Tu S, Gao S, Shao A, Sheng J. The epidemiological and clinical features of COVID-19 and lessons from this global infectious public health event. J Infect. (2020) doi: 10.1016/j.jinf.2020.04.011. [Epub ahead of print].
88. Uchida D, Fujimoto A, Yamazoe T, Yamamoto T, Enok H. Seizure frequency can be reduced by changing intracranial pressure: A case report in drug-resistant epilepsy. Epilepsy Behav Case Rep. (2018) 10:14–17. doi: 10.1016/j.ebcr.2017.12.005
89. Ogunlaja OI, Cowan R. Subarachnoid hemorrhage and headache. Curr Pain Headache Rep. (2019) 23:44. doi: 10.1007/s11916-019-0785-x
90. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033–4. doi: 10.1016/S0140-6736(20)30628-0
91. Li Y, Wang M, Zhou Y, Chang J, Xian Y, Mao L, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. SSRN Electr J. (2020) doi: 10.2139/ssrn.3550025. [Epub ahead of print].
92. Nakamura K, Saito K, Hara Y, Aoyagi T, Kitakawa K, Abe Y, et al. Severe epidemic myalgia with an elevated level of serum interleukin-6 caused by human parechovirus type 3: a case report and brief review of the literature. BMC Infect Dis. (2018) 18:381. doi: 10.1186/s12879-018-3284-5
93. Tanaka T, Narazaki M, Kishimoto T. IL-6 in Inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
94. Marks M, Marks JL. Viral arthritis. Clin Med. (2016) 16:129–34. doi: 10.7861/clinmedicine.16-2-129
95. World Health Organization Report. Multisystem Inflammatory Syndrome in Children and Adolescents With COVID-19: Scientific Brief. (2020) reference number: WHO/2019-CoV/Sci /2020.1.
96. Pinzon RT, Wijaya VO, Buana RB, Jody AA, Nunsio PN. Neurologic characteristics in Coronavirus Disease 2019 (COVID-19): a systematic review and meta-analysis. Front Neurol. (2020) 194:105921. doi: 10.3389/fneur.2020.00565
Keywords: COVID-19, symptoms, myalgia, taste impairment, anosmia, cytokine storm, headache, muscle weakness
Citation: Abdullahi A, Candan SA, Abba MA, Bello AH, Alshehri MA, Afamefuna Victor E, Umar NA and Kundakci B (2020) Neurological and Musculoskeletal Features of COVID-19: A Systematic Review and Meta-Analysis. Front. Neurol. 11:687. doi: 10.3389/fneur.2020.00687
Received: 06 May 2020; Accepted: 08 June 2020;
Published: 26 June 2020.
Edited by:
Rosanna Cardani, IRCCS Policlinico San Donato, ItalyReviewed by:
Carmelo Rodolico, University of Messina, ItalyChiara Terracciano, Gugliemo da Saliceto Hospital, Italy
Copyright © 2020 Abdullahi, Candan, Abba, Bello, Alshehri, Afamefuna Victor, Umar and Kundakci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Auwal Abdullahi, aabdullahi.pth@buk.edu.ng
 Auwal Abdullahi
Auwal Abdullahi Sevim Acaroz Candan
Sevim Acaroz Candan Muhammad Aliyu Abba
Muhammad Aliyu Abba Auwal Hassan Bello5
Auwal Hassan Bello5 Mansour Abdullah Alshehri
Mansour Abdullah Alshehri