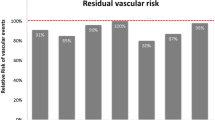
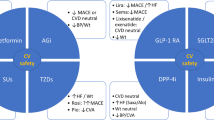
Abstract
Diabetes mellitus is a major risk factor for cardiovascular (CV) disease. Conversely, CV disease is responsible for a majority of the deaths in patients with diabetes. Many drug trials have concentrated on blood glucose (hemoglobin A1c) reduction. This strategy, while reducing microvascular outcomes like nephropathy and neuropathy, has little or no effect on reducing macrovascular events like heart attack, stroke, and heart failure. It has been postulated that hypoglycemia may counterbalance some of the beneficial effects of anti-hyperglycemic agents, but this is not proven. Further, trial evidence for thiazolidinediones (rosiglitazone and pioglitazone) showed increased risk of heart failure and raised concerns about increased myocardial infarction. This heightened awareness of potentially harmful CV effects of otherwise effective hypoglycemic drugs resulted in regulatory mandates for CV outcome trials to ascertain the safety of newer anti-hypoglycemic agents appearing on the market. Three new classes of anti-hyperglycemic agents have been introduced in recent years. While dipeptidyl peptidase-4 (DPP-4) inhibitors exhibited increased heart failure hospitalization in the SAVOR-TIMI 53 trial evaluating saxagliptin and in the secondary analysis of the EXAMINE trial for alogliptin, the effects of glucagon-like peptide-1 (GLP-1) analogs and sodium-glucose co-transporter-2 (SGLT2) inhibitors on CV outcomes in diabetes have largely been positive. The LEADER and SUSTAIN-6 trials evaluating the safety and efficacy of the GLP-1 analogs liraglutide and semaglutide, respectively, showed a statistically significant reduction in the primary outcome (major adverse cardiac events [MACE]: CV death, myocardial infarction, and stroke) and the secondary combined outcome when compared to placebo. Results of the TECOS trial for sitagliptin were, however, neutral (no net CV benefit or harm), questioning the class effect of GLP-1 analogs. Results of the SGLT2 inhibitor trials were more uniform. While EMPA-REG (evaluating empagliflozin) and CANVAS (evaluating canagliflozin) showed a reduction in the MACE end point, dapagliflozin had a net neutral effect on MACE in DECLARE-TIMI 58. All three SGLT2 inhibitors, however, showed a significant reduction in heart failure hospitalizations. Although initially designed to keep potentially harmful anti-hyperglycemic agents off the market, the CV outcome trials have provided clinicians with a new set of anti-hyperglycemic drugs with proven CV benefit in patients with diabetes and CV disease, thus expanding the field of CV secondary prevention. There is a need to inculcate GLP-1 analogs and SGLT2 inhibitors that reduce major CV events and heart failure hospitalizations (alongside lifestyle management and metformin) in the treatment of patients with diabetes and CV disease.
Similar content being viewed by others
References
Benjamin EJ, Blaha MJ, Chiuve SE, American Heart Association Statistics Committee and Stroke Statistics Subcommittee, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–603.
Saydah SH, Eberhardt MS, Loria CM, Brancati FL. Age and the burden of death attributable to diabetes in the United States. Am J Epidemiol. 2002;156(8):714–9.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Wong MG, Perkovic V, Chalmers J, ADVANCE-ON Collaborative Group, et al. Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care. 2016;39(5):694–700.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–98.
Gerstein HC, Miller ME, Ismail-Beigi F, et al. Effects of intensive glycaemic control on ischaemic heart disease: analysis of data from the randomised, controlled ACCORD trial. Lancet. 2014;384:1936–41.
Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–206.
Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–36.
Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes—an interim analysis. N Engl J Med. 2007;357:28–38.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71.
Department of Health and Human Services, Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf. Accessed 15 Jul 2017.
European Medicines Agency guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. London: Committee for Medicinal Products for Human Use; 2012.
Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs—insights from the rosiglitazone experience. N Engl J Med. 2013;369:1285–7.
Gupta A, Jelinek HF, Al-Aubaidy H. Glucagon like peptide-1 and its receptor agonists: their roles in management of type 2 diabetes mellitus. Diabetes Metab Syndr. 2017;11(3):225–30.
Zhong J, Maiseyeu A, Davis SN, Rajagopalan S. DPP4 in cardiometabolic disease: recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ Res. 2015;116:1491–504.
Scirica BM, Bhatt DL, Braunwald E, SAVOR-TIMI 53 Steering Committee and Investigators, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.
White WB, Cannon CP, Heller SR, EXAMINE Investigators, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35.
Green JB, Bethel MA, Armstrong PW, TECOS Study Group, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42.
Zannad F, Cannon CP, Cushman WC, EXAMINE Investigators, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385(9982):2067–76.
FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM493965.pdf. Accessed 21 Jul 17.
Cimmaruta D, Maiorino MI, Scavone C, et al. Efficacy and safety of insulin-GLP-1 receptor agonists combination in type 2 diabetes mellitus: a systematic review. Expert Opin Drug Saf. 2016;15:77–83.
Bunck MC, Corner A, Eliasson B, et al. Effects of exenatide on measures of beta-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care. 2011;34:2041–7.
Von Scholten BJ, Lajer M, Goetze JP, Persson F, Rossing P. Time course and mechanisms of the anti-hypertensive and renal effects of liraglutide treatment. Diabet Med. 2015;32:343–52.
Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–9.
Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5.
Lonborg J, Vejlstrup N, Kelbaek H, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–9.
Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor dependent and -independent pathways. Circulation. 2008;117:2340–50.
Chilton R, Wyatt J, Nandish S, Oliveros R, Lujan M. Cardiovascular comorbidities of type 2 diabetes mellitus: defining the potential of glucagon like peptide-1-based therapies. Am J Med. 2011;124:S35–53.
Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24:15–30.
Pfeffer MA, Claggett B, Diaz R, ELIXA Investigators, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57.
Marso SP, Daniels GH, Brown-Frandsen K, LEADER Steering Committee, LEADER Trial Investigators, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Marso SP, Bain SC, Consoli A, SUSTAIN-6 Investigators, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44.
Holman RR, Bethel MA, Mentz RJ, EXSCEL Study Group, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39.
Gerstein HC, Colhoun HM, Dagenais GR, REWIND Trial Investigators, et al. Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab. 2017. https://doi.org/10.1111/dom.13028 (Epub ahead of print).
Sattar N, Petrie MC, Zinman B, Januzzi JL Jr. Novel diabetes drugs and the cardiovascular specialist. J Am Coll Cardiol. 2017;69(21):2646–56.
Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515–31.
Madaan T, Akhtar M, Najmi AK. Sodium glucose cotransporter 2 (SGLT2) inhibitors: current status and future perspective. Eur J Pharm Sci. 2016;93:244–52.
Sanchez RA, Sanabria H, de Los Santos C, Ramirez AJ. Incretins and selective renal sodium-glucose co-transporter 2 inhibitors in hypertension and coronary heart disease. World J Diabetes. 2015;6:1186–97.
Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J. 2015;36:2288–96.
Wanner C, Inzucchi SE, Lachin JM, EMPA-REG OUTCOME Investigators, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34.
Zinman B, Wanner C, Lachin JM, EMPA-REG OUTCOME Investigators, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME trial. Eur Heart J. 2016;37:1526–34.
https://clinicaltrials.gov/ct2/show/NCT03057977. Accessed 9 Oct 2017.
Neal B, Perkovic V, Mahaffey KW, CANVAS Program Collaborative Group, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Wiviott SD, Raz I, Bonaca MP, et al. The design and rationale for the Dapagliflozin Effect on Cardiovascular Events (DECLARE)–TIMI 58 Trial. Am Heart J. 2018;200:83–9.
Wiviott SD, Raz I, Bonaca MP, DECLARE–TIMI 58 Investigators, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018. https://doi.org/10.1056/nejmoa1812389 (Epub ahead of print).
Kosiborod M, Cavender MA, Fu AZ, CVD-REAL Investigators and Study Group, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation. 2017;136(3):249–59.
Kosiborod M, Lam CSP, Kohsaka S, CVD-REAL Investigators and Study Group, et al. Lower Cardiovascular risk associated with SGLT-2i in > 400,000 patients: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71(23):2628–39.
FDA Drug Safety Communication: FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR) [news release]. FDA’s website. https://www.fda.gov/Drugs/DrugSafety/ucm557507.htm?source = govdelivery&utm_medium = email&utm_source = govdelivery. Accessed 8 Oct 2017.
Cardiovascular disease and risk management. Standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Suppl. 1):S86–104.
Funding
No external funding was used in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Deedwania has served as a consultant/speaker for Janssen Pharmaceuticals. Dr. Acharya has no potential conflicts of interest that might be relevant to this manuscript.
Rights and permissions
About this article
Cite this article
Deedwania, P., Acharya, T. Cardiovascular Protection with Anti-hyperglycemic Agents. Am J Cardiovasc Drugs 19, 249–257 (2019). https://doi.org/10.1007/s40256-019-00325-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-019-00325-9