-
PDF
- Split View
-
Views
-
Cite
Cite
Kento Yoshikawa, Takashi Umekawa, Shintaro Maki, Michiko Kubo, Masafumi Nii, Kayo Tanaka, Hiroaki Tanaka, Kazuhiro Osato, Yuki Kamimoto, Eiji Kondo, Kenji Ikemura, Masahiro Okuda, Kan Katayama, Takekazu Miyoshi, Hiroshi Hosoda, Ning Ma, Toshimichi Yoshida, Tomoaki Ikeda, Tadalafil Improves L-NG-Nitroarginine Methyl Ester-Induced Preeclampsia With Fetal Growth Restriction-Like Symptoms in Pregnant Mice, American Journal of Hypertension, Volume 31, Issue 1, January 2018, Pages 89–96, https://doi.org/10.1093/ajh/hpx130
Close - Share Icon Share
Abstract
We investigated the efficacy and mechanisms of tadalafil, a selective phosphodiesterase 5 inhibitor, in treating preeclampsia (PE) with fetal growth restriction (FGR) using L-NG-nitroarginine methyl ester (L-NAME)-induced PE with FGR in pregnant mice as our experimental model.
C57BL/6 mice were divided into 2 groups 11 days postcoitum (d.p.c.). A control group of dams (C dam) received 0.5% carboxymethylcellulose (CMC). A L-NAME-treated group received 1 mg/ml L-NAME dissolved in CMC. The L-NAME-treated dams were divided into 2 subgroups 13 d.p.c. One subgroup continued to receive L-NAME (L dams). The other subgroup received L-NAME with 0.08 mg/ml tadalafil suspended in CMC (TL dams). Maternal systolic blood pressure (SBP) and proteinuria were assessed 16 d.p.c. Fetal weight was recorded, and placentas and maternal kidneys were collected 17 d.p.c.
Maternal SBP, proteinuria, and fetal weight were improved for TL dams compared to L dams. The placental concentration of placental growth factor (PlGF) was higher for TL dams than for the C and L dams. The placental maternal blood sinuses of L dams were narrower than those of C dams, but those of TL dams improved to a similar width as C dams. Glomerular oxidative stress was ameliorated in TL dams compared to L dams.
Tadalafil dilates the placental maternal blood sinuses, which leads to increase PlGF production, and contributes to facilitate fetal growth and improve maternal SBP. Moreover, tadalafil ameliorates glomerular damage by reducing oxidative stress. These results suggest that tadalafil is a candidate for treatment of PE with FGR.
Preeclampsia (PE) is often associated with fetal growth restriction (FGR), since both PE and FGR are disorders rooted in defects in early placental development.1 The failure of trophoblast invasion and remodeling of the uterine spiral arteries leads to high-resistance in uterine circulation, causing a reduction in blood flow and placental ischemia. Placental ischemia lead to excess release of soluble fms-like tyrosine kinase-1 (sFlt-1) from the placenta which sequester circulating placental growth factor (PlGF), resulting in FGR, endothelial and, specifically, renal dysfunction.2
Nitric oxide (NO) produced by NO synthases (NOS) regulates vascular tone in the placenta. Placental vessels express molecular mediators of the NO-dependent pathway, including cGMP-specific phosphodiesterases (PDE).3 Because inhibitors of PDE5, which is a cyclic guanosine monophosphate (cGMP)-specific PDE, exert their pharmacological action by dilating arteries and increasing blood flow in erectile dysfunction and pulmonary hypertension,4 recent studies have suggested a potential therapeutic role for PDE5 inhibitors in treating PE.5 Tadalafil, a selective PDE5 inhibitor, has been used to treat pulmonary hypertension in pregnant women. Tadalafil has a relatively short time to onset of action and a much longer half-life than sildenafil.4 We have recently reported a potential therapeutic effect for tadalafil on PE and FGR in small clinical trials.6–9 Our clinical data showed that tadalafil treatment improved perinatal outcome in FGR by modulating fetal growth and ameliorated the symptoms of PE.7,8 Interestingly, maternal serum sFlt-1 and PlGF levels were immediately improved after tadalafil administration in the case of PE with FGR.8 These findings made us hypothesize that tadalafil may improve PE with FGR by modulating the production of sFlt-1 and PlGF in the placenta. The aim of this study was to investigate the potential efficacy and mechanisms of tadalafil in treating PE with FGR using L-NG-nitroarginine methyl ester (L-NAME)-induced mouse model of PE with FGR.
METHODS
This study was conducted in accordance with the principles and procedures outlined by the Ethics Committee for Animal Research of the Mie University Graduate School of Medicine.
Nineteen pregnant C57BL/6 mice (CLEA Japan, Tokyo, Japan) were purchased 9 days postcoitum (d.p.c.). Food and water were available ad libitum, and the body weight (BW), as well as food and water intake by the dams were assessed daily. Dams were matched by weight and divided into 2 groups 11 d.p.c. (Supplementary Table S1). A control group of dams (n = 6) received 0.5% carboxymethylcellulose (CMC; Wako Pure Chemical Industries, Osaka, Japan) dissolved in drinking water (C dam). An L-NAME treated group (n = 13) received 1 mg/ml L-NAME (Cayman Chemical, Ann Arbor, MI, US) dissolved in 0.5% CMC. The L-NAME-treated dams were matched by weight and systolic blood pressure (SBP), and were divided into 2 subgroups 13 d.p.c. (Supplementary Table S1 and Figure 1a, left panel). One subgroup continued to receive only L-NAME (L dam, n = 7) while the other subgroup received L-NAME with a 0.08 mg/ml tadalafil (Cayman Chemical) suspended in 0.5% CMC (TL dam, n = 6).10 The concentrations of tadalafil in CMC and the maternal serum were confirmed by high-performance liquid chromatography analysis (HPLC).11,12 The limit of detection of tadalafil by HPLC was 3 ng/ml. L-NAME and tadalafil dosages were calculated on the basis of water intake volumes. In preliminary experiments, 6 nonpregnant female mice were randomly divided into 3 groups and treated with tadalafil suspended in 0.5% CMC (0.02, 0.08, and 0.2 mg/ml). The serum concentrations of tadalafil could not be detected by HPLC in the group treated by 0.02 mg/ml of tadalafil (the average dose of tadalafil was 6.1 mg/kg BW/day). The BW of 1 mouse in the group treated by 0.2 mg/ml of tadalafil reduced after 4 days of tadalafil treatment (the average dose of tadalafil was 57.0 mg/kg BW/day). Because the serum tadalafil concentrations (10.3 ng/ml and 21.3 ng/ml) were almost consistent with the previous study reported by García-Barroso et al.13 and the BWs did not decrease in the group treated by 0.08 mg/ml of tadalafil (the average dose of tadalafil was 18.5 mg/kg BW/day), we decided the concentration of tadalafil to be 0.08 mg/ml. The dams were sacrificed 17 d.p.c. Resorption rate was calculated as the ratio between resorbed fetuses to normal implantation sites.14 Wet weights of the fetus and placenta were recorded. Placentas and maternal kidneys were collected for further analysis.
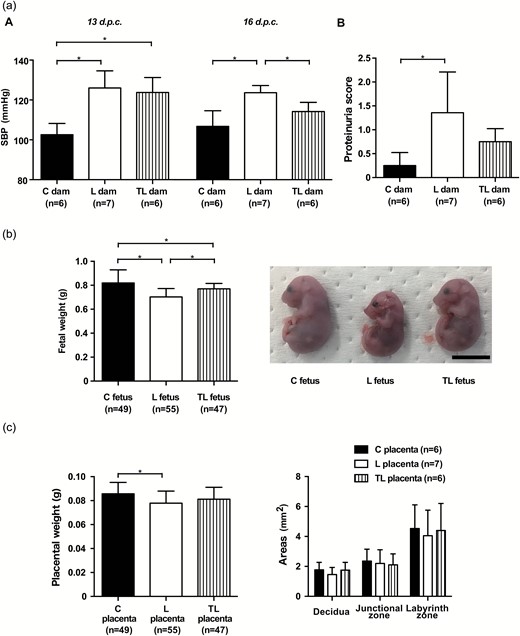
Maternal, fetal, and placental characteristics. (a) Maternal characteristics. (A) Mean SBP 13 d.p.c. and 16 d.p.c. for C dams (n = 6), L dams (n = 7), and TL dams (n = 6). (B). Proteinuria score 16 d.p.c. in C dams (n = 6), L dams (n = 7), and TL dams (n = 6). (b) Fetal characteristics. Fetal body weight of the C fetus (n = 49), L fetus (n = 55), and TL fetus (n = 47), and gross appearance of the fetus in each group 17 d.p.c. Scale bar: 1 cm. (c) Placental characteristics. Placental weight of the C placenta (n = 49), L placenta (n = 55), TL placenta (n = 47), and area of the 3 placental layers (decidua, junctional zone, labyrinth zone) of the C placenta (n = 6), L placenta (n = 7), and TL placenta (n = 6) 17 d.p.c. The area of 3 placental layers was measured as described in the Supplementary Methods section in Supplementary Material. Values are presented as mean ± SD. Asterisks show statistically significant differences (P < 0.05) between groups indicated by square brackets as determined by 1-way ANOVA followed by Bonferroni’s post-hoc test. C fetus and C placenta: fetus and placenta from C dams. L fetus and L placenta: fetus and placenta from L dams. TL fetus and TL placenta: fetus and placenta from TL dams. Abbreviations: ANOVA, analysis of variance; d.p.c., days postcoitum; SBP, systolic blood pressure.
Alternatively, to confirm the efficacy of treatment by 0.08 mg/ml of tadalafil, 16 pregnant C57BL/6 mice were assigned to L-NAME treatment alone (n = 8) or L-NAME with 0.08 mg/ml of tadalafil treatment (n = 8) by the similar procedure above to examine urinary excretion of cGMP. Three days after the initiation of tadalafil treatment, urine was collected from both groups of pregnant mice placed in metabolic cages (KN-646B, Natsume Seisakusyo, Tokyo, Japan) for 24 hours.
An additional set of experiments was conducted to examine the impact of tadalafil treatment on normal pregnancy. Pregnant mice were assigned to no treatment (n = 6) or tadalafil treatment (n = 3) by the similar procedure above. A no-treatment group received 0.5% CMC, and a tadalafil-treated group received 0.08 mg/ml of tadalafil suspended in 0.5% CMC from 13 to 17 d.p.c. The dams were sacrificed 17 d.p.c.
The detailed methods of the assessment of maternal SBP and proteinuria, the measurement of PlGF, sFlt-1, cGMP and creatinine levels, and histopathological and immunohistochemical analysis of the placenta and the kidney are available as Supplementary Material.
Statistical analysis
All values are presented as means ± SD. All statistical tests were carried out using GraphPad Prism6 (GraphPad, San Diego, CA). One-way analysis of variance followed by Bonferroni’s post-hoc test was used for multiple comparisons among the L dams, the TL dams, and the C dams. We used an unpaired Student’s t-test when comparing urinary excretion of cGMP between the L-NAME treatment group and the L-NAME with tadalafil treatment group. An unpaired Student’s t-test was also used when comparing between the no-treatment group and the tadalafil-treated group. P values <0.05 were considered statistically significant.
RESULTS
L-NAME with tadalafil treatment improved L-NAME-induced hypertension and proteinuria in pregnant mice
The maternal BW 17 d.p.c. and the maternal weight gain from 13 to 17 d.p.c. of the L dam were both significantly lower than those of the C dam, whereas there were no significant differences between those of the C dam and the TL dam (Supplementary Table S1). The maternal daily food intake did not differ significantly among the 3 groups. The average dose of L-NAME was 302.9 ± 32.7 mg/kg BW/day. The average dose of tadalafil was 23.1 ± 1.3 mg/kg BW/day, and the concentration of tadalafil in the maternal serum was 8.2 ± 3.2 ng/ml.
The mean SBP measured by tail-cuff methods was significantly higher for the L and the TL dams compared to C dams 13 d.p.c. (C dams, 102.6 ± 5.7 mm Hg; L dams, 126.0 ± 8.6 mm Hg; TL dams, 123.8 ± 7.5 mm Hg; Figure 1a, A, left panel). L dams had a significantly higher mean SBP than C dams 16 d.p.c., but the mean SBP improved significantly for TL dams in comparison with L dams (C dams, 106.8 ± 7.9 mm Hg; L dams, 123.7 ± 3.6 mm Hg; TL dams, 114.2 ± 4.6 mm Hg; Figure 1a, A, right panel). The proteinuria score assessed by a dipstick method was significantly higher for L dams than for C dams 16 d.p.c. (Figure 1a, B). In contrast, there was no significant difference in the proteinuria score between C dams and TL dams (C dams, 0.25 ± 0.27; L dams, 1.36 ± 0.85; TL dams, 0.75 ± 0.27; Figure 1a, B).
Effects of L-NAME and tadalafil treatment on fetal and placental development
Litter sizes did not differ significantly among the 3 groups of dams (C dams, 8.2 ± 1.0; L dams, 7.9 ± 0.9; TL dams, 7.8 ± 0.8; P = 0.77). The resorption rate of the L dam was about 2 times higher than that of the C dam and the TL dam, but this was not significantly different (C dams, 1.7 ± 4.1%; L dams, 3.4 ± 5.8%; TL dams, 1.9 ± 4.5%; P = 0.79). Fetal BW 17 d.p.c. was significantly lower for L dams than C dams. In contrast, fetal BW improved significantly for TL dams compared to L dams (C dams, 0.82 ± 0.11 g; L dams, 0.70 ± 0.07 g; TL dams, 0.77 ± 0.05 g; Figure 1b). Placental weight was significantly lower for L dams than for C dams, but there were no significant differences between the C and TL dams (C dams, 0.086 ± 0.010 g; L dams, 0.078 ± 0.010 g; TL dams, 0.081 ± 0.010 g; Figure 1c, left panel). The areas of the three placental layers (decidua, junctional zone, labyrinth zone) did not differ significantly among the 3 groups (P = 0.48, P = 0.87, and P = 0.88, respectively; Figure 1c, right panel).
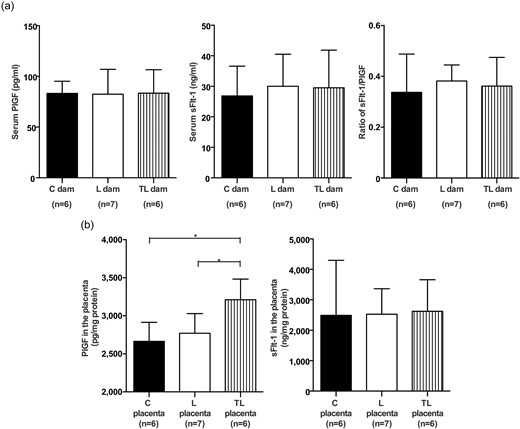
Increased PlGF protein concentration in L-NAME with tadalafil-treated mice
There were no significant differences in the maternal serum PlGF (C dams, 83.2 ± 12.0 pg/ml; L dams, 82.4 ± 24.5 pg/ml; TL dams, 83.5 ± 23.1 pg/ml) and sFlt-1 concentrations (C dams, 26.9 ± 9.7 ng/ml; L dams, 30.0 ± 10.5 ng/ml; TL dams, 29.5 ± 12.4 ng/ml) 17 d.p.c. (Figure 2a). The ratio of sFlt-1/PlGF was not significantly different among the 3 groups (C dams, 0.34 ± 0.15; L dams, 0.38 ± 0.06; TL dams, 0.36 ± 0.11; Figure 2a). Notably, the PlGF concentration in the placenta of TL dams was significantly higher than in the C and L dams (C dams, 2,664 ± 251.1 pg/mg protein; L dams, 2,770 ± 258.4 pg/mg protein; TL dams, 3,211 ± 271.3 pg/mg protein; Figure 2b, left panel). The sFlt-1 concentration in the placenta was not significantly different among the 3 groups (C dams, 2,489 ± 1,807 ng/mg protein; L dams, 2,528 ± 836.5 ng/mg protein; TL dams, 2,621 ± 1,038 ng/mg protein; Figure 2b, right panel).
PlGF and sFlt-1 concentrations in maternal serum and the placenta. (a) PlGF and sFlt-1 concentrations in maternal serum and the ratio of sFlt-1/PlGF of C dams (n = 6), L dams (n = 7), and TL dams (n = 6) 17 d.p.c. (b) PlGF and sFlt-1 concentrations of the C placenta (n = 6), L placenta (n = 7), and TL placenta (n = 6) 17 d.p.c. Values are presented as mean ± SD. Asterisks show statistically significant differences (P < 0.05) between groups indicated by square brackets as determined by 1-way ANOVA followed by Bonferroni’s post-hoc test. C placenta: placenta from C dams. L placenta: placenta from L dams. TL placenta: placenta from TL dams. Abbreviations: ANOVA, analysis of variance; d.p.c., days postcoitum; sFlt-1, soluble fms-like tyrosine kinase-1; PlGF, placental growth factor.
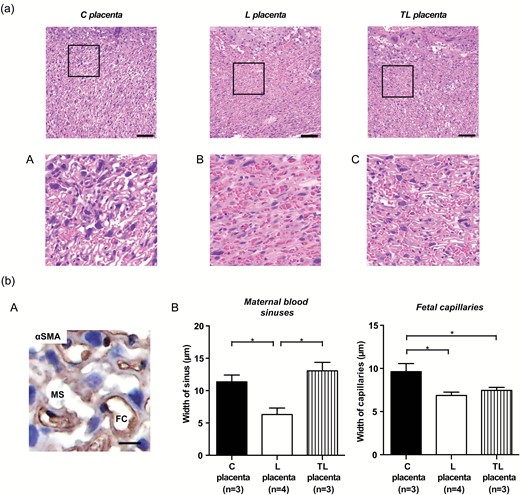
L-NAME treatment narrowed maternal blood sinuses in the labyrinth zone, whereas L-NAME with tadalafil treatment widened them
HE staining showed that maternal and fetal blood spaces in the labyrinth zone of the placenta were narrowed in L dams, but these spaces in TL dams were widened (Figure 3a). Histological analysis by alpha-smooth muscle actin staining revealed that the width of the maternal blood sinuses was significantly decreased for L dams compared to C dams, but significantly improved for TL dams compared to L dams (C dams, 11.4 ± 1.1 µm; L dams, 6.3 ± 1.0 µm; TL dams, 13.1 ± 1.3 µm; Figure 3b, B, left panel). In contrast, fetal capillary width was significantly lower for the L and the TL dams compared to C dams (C dams, 9.6 ± 0.9 µm; L dams, 6.9 ± 0.4 µm; TL dams, 7.5 ± 0.4 µm; Figure 3b, B, right panel).
Histological analysis of the placenta. (a) Representative HE staining images of the labyrinth zone in the placenta from C dams (C placenta), L dams (L placenta), and TL dams (TL placenta). Scale bars: 100 µm. The areas in the small rectangles are shown at higher magnification in the row below (A–C). (b). Histological analysis of maternal blood sinus and fetal capillary in the labyrinth zone. (A) Representative images of maternal blood sinus (αSMA negative) and fetal capillary (αSMA positive) in the labyrinth zone. Scale bar: 10 µm. Nuclei were counterstained with hematoxylin. (B) Width of the maternal blood sinuses and the fetal capillaries in the labyrinth zone of the C placenta (n = 3), L placenta (n = 4), and TL placenta (n = 3). Asterisks show statistically significant differences (P < 0.05) between groups indicated by square brackets as determined by 1-way ANOVA followed by Bonferroni’s post-hoc test. C placenta: placenta from C dams. L placenta: placenta from L dams. TL placenta: placenta from TL dams. Abbreviations: ANOVA, analysis of variance; FC, fetal capillary; MS, maternal blood sinus; αSMA, alpha-smooth muscle actin.
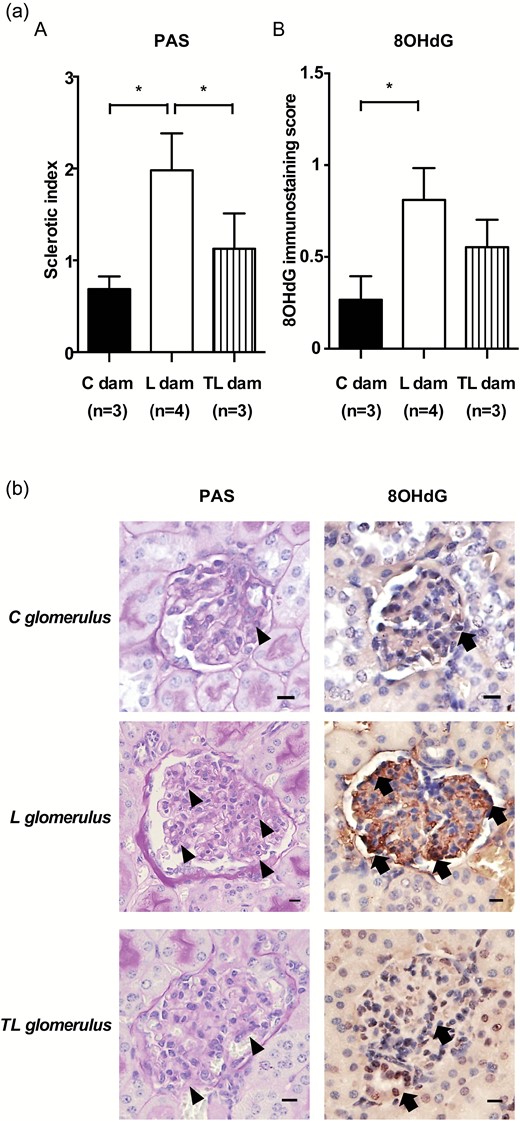
Treatment with L-NAME and tadalafil improved L-NAME-induced renal injury
Glomerular sclerotic indices assessed by PAS staining were significantly higher for L dams compared with C dams. In contrast, glomerular sclerotic indices were significantly improved for TL dams compared with L dams (C dams, 0.69 ± 0.14; L dams, 1.98 ± 0.40; TL dams, 1.13 ± 0.38; Figure 4a). Interestingly, 8-OHdG expression, which is indicative of DNA damage due to oxidative stress, was prominent in the glomerulus with high sclerotic indices of L dams, but was weak in the glomerulus with low sclerotic indices of C dams and TL dams. The glomerular 8-OHdG immunostaining score was significantly higher for the L dams than for the C dams. In contrast, there was no significant difference between the C dams and the TL dams (C dams, 0.27 ± 0.13; L dams, 0.81 ± 0.17; TL dams, 0.55 ± 0.15; Figure 4a, B and b).
Histological analysis of the kidney. (a) (A) The sclerotic index of the glomerulus and (B) the glomerular 8-OHdG immunostaining score of C dams (n = 3), L dams (n = 4), and TL dams (n = 4). Asterisks show statistically significant differences (P < 0.05) between groups indicated by square brackets as determined by 1-way ANOVA followed by Bonferroni’s post-hoc test. (b) PAS staining and 8OHdG immunostaining images of the glomerulus. 8-OHdG expression (arrows) was prominent in the glomerulus of L dams (arrow heads indicate glomerular sclerosis; sclerotic index score = 3), but it was weak in the glomerulus of C dams (sclerotic index score = 1) and TL dams (sclerotic index score = 1). Scale bars: 10 µm. C glomerulus: glomerulus in the kidney of C dams. L glomerulus: glomerulus in the kidney of L dams. TL glomerulus: glomerulus in the kidney of TL dams. Nuclei were counterstained with hematoxylin. Abbreviation: ANOVA, analysis of variance;
Tadalafil treatment increased urinary cGMP excretion
Urinary excretion of cGMP was significantly elevated in the L-NAME with tadalafil treatment group compared with the L-NAME treatment group (20.2 ± 5.0 nmol/mg creatinine (n = 8) vs. 15.3 ± 3.4 nmol/mg creatinine (n = 8), P < 0.05).
Tadalafil treatment in normal pregnant mice
The maternal BW 17 d.p.c., the maternal weight gain from 13 to 17 d.p.c., the maternal daily food intake, and the mean SBP 16 d.p.c. did not differ significantly between the no-treatment group and the tadalafil-treated group (Supplementary Figure S1A–D). There were no significant differences in fetal BW and placental weight between the 2 groups (Supplementary Figure S1E and F). The PlGF and sFlt-1 concentrations in the placenta did not differ significantly between the 2 groups (Supplementary Figure S1G and H).
DISCUSSION
The role of NO appears to support the low basal tone in the fetoplacental circulation keeping placental vessels in a vasodilated state and attenuating the effects of vasoconstrictors.15 Our results indicate that tadalafil treatment contributes to improve PE signs and facilitate fetal growth in the context of mechanisms associated with NO signaling. In the previous reports, chronic inhibition of NOS using L-NAME leads to the development of an animal model of PE characterized by hypertension and proteinuria with FGR.16 Sildenafil, which is also a selective PDE5 inhibitor, have been shown to affect maternal hypertension, but not FGR induced by L-NAME treatment in pregnant rats, except for one report by Baijnath et al.17–20 Baijnath et al. demonstrated that L-NAME-induced FGR was improved by sildenafil treatment from 4 d.p.c. to 8 d.p.c. but not from 8 d.p.c. to 14 d.p.c. Because chorioallantoic attachment occurs at 8 d.p.c., and the mature circulatory pattern of maternal blood through the placenta is established by 10 d.p.c. in mouse placenta,21,22 L-NAME treatment was started at 11 d.p.c. in this study. In considering the development of fetoplacental circulation in rodents, the effect of sildenafil on fetal growth associated with placental blood flow via an NO-dependent pathway was not manifested. Burke et al. demonstrated that reduction in uterine artery resistance index and histological changes in the placenta of sFlt-1 adenovirus injection with sildenafil group.24 Because the sildenafil treatment was started at the same day with sFlt-1 adenovirus injection, we think it is difficult to say that the findings induced by sFlt-1 adenovirus injection were ameliorated by sildenafil treatment. In our study, we initiated tadalafil treatment after confirming that the mean maternal SBP was higher for the L-NAME-treated dams compared to the control dams. In addition, we examined placentas from the L-NAME-treated dams and the control dams 13 d.p.c., and histological observation of the placenta showed that blood spaces in the labyrinth zone of the placenta were narrowed by L-NAME treatment (data not shown). Thus, we can safely presume that tadalafil treatment dilates the blood spaces in the labyrinth zone. There is a possibility that the difference in fetal growth between dams treated with sildenafil and tadalafil may be caused by selectivity for PDE5, because sildenafil has a 10-fold greater selectivity for PDE5 over PDE6, whereas tadalafil is 700-fold more potent against PDE5 than PDE6.23,25 We also demonstrated that the width of the maternal blood sinuses in the labyrinth zone of the placenta improved to a similar level as the control placenta in mice treated with L-NAME and tadalafil. Moreover, L-NAME with tadalafil increased the PlGF concentration in the placenta, whereas there were no significant changes in the placental sFlt-1 concentration. PlGF has been reported to be downregulated in response to low oxygen levels in trophoblasts26,27 and to have a vasodilatory effect mediated by the release of NO.28 Gu et al. suggested that PlGF production is more sensitive toward oxygen levels than sFlt-1 production in trophoblasts.29 Our unpublished data indicated that, whereas there was no significant difference in HIF-1α expression between the C placenta and the L placenta, the expression of HIF-1α were significantly lower for the TL placenta than for the L placenta (unpublished data by K.Y., T.U., and T.I.). We demonstrated that urinary excretion of cGMP was significantly elevated by tadalafil treatment in the present study. Based on this study and on previous reports, the amplification of NO signaling induced by PDE5 inhibition with tadalafil treatment leads to an increase in PlGF production via ameliorating placental hypoxia by dilating the maternal blood sinuses, and contributes to improve PE signs and facilitate fetal growth. Lambert et al. reported that tadalafil contributed to facilitate fetal growth, which is consistent with our results.30 They showed that tadalafil reduced halogen inhalation-induced inflammation in the placenta. Yano et al. demonstrated that PlGF plays an important role in the response to inflammation.31 Taken together, it is possible that tadalafil may facilitate fetal growth via anti-inflammatory effects mediated through PlGF signaling. However, further studies are needed. Tadalafil treatment showed no effect on maternal SBP, fetal weight, and PlGF concentrations in the placenta in normal pregnant mice in this study. Itani et al. demonstrated that hypoxic condition increased the protein expression of PDE5, whereas sildenafil treatment under normoxic condition showed no effect on PDE5 expression,32 which may explain our results in normal pregnant mice.
L-NAME treatment has been reported to cause glomerular damage consisting of glomerulosclerosis.33,34 We showed here that treatment with L-NAME and tadalafil not only improved L-NAME-induced glomerulosclerosis but also ameliorated oxidative stress in the glomeruli. Although previous studies reported a pathophysiological role for excess circulating sFlt-1 in PE, the sFlt-1 levels in the maternal serum and the placenta were unchanged in this study.35 These findings indicate that glomerular damage may be caused by NOS inhibition directly in the kidney in the present model. Burke et al. demonstrated that sildenafil treatment ameliorated vascular oxidative stress in pregnant mice that had been infected with sFlt-1 adenovirus.24 Tain et al. reported that the antioxidant vitamin E reduced glomerulosclerosis and preserved renal NOS abundance.36 This may explain our findings that tadalafil treatment ameliorates glomerular damage caused by NOS inhibition, at least in part by reducing oxidative stress.
In summary, we demonstrated that tadalafil treatment dilates the maternal blood sinuses, which leads to increase PlGF production, and contributes to facilitate fetal growth and improve maternal SBP. Moreover, tadalafil treatment ameliorates glomerular damage caused by NOS inhibition by also reducing oxidative stress. These results suggest that tadalafil is a candidate for treatment of PE with FGR.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
We thank Ms. Miyuki Namikata for her assistance with histological procedures. This study was supported by the Takeda Science Foundation.
REFERENCES