Abstract
Purpose
The phosphodiesterase type 5 inhibitor tadalafil is approved for the treatment of signs and symptoms of benign prostatic hyperplasia (BPH). While tadalafil significantly improves overall lower urinary tract symptoms suggestive of BPH (LUTS/BPH), improvements in nocturia were not significant in individual studies. We therefore sought to further assess nocturia based on data integrated from four tadalafil registrational studies.
Methods
Data were integrated from four randomized, placebo-controlled, double-blind, 12-week registrational studies of tadalafil for LUTS/BPH. Nocturia was assessed as nighttime voiding frequency using the International Prostate Symptom Score question 7 (IPSS Q7). Efficacy results were analyzed using analysis of covariance.
Results
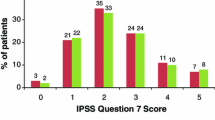
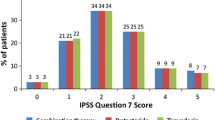
For the tadalafil 5 mg once daily (N = 752) and placebo (N = 748) groups, baseline characteristics were well balanced, and the overall severity of nocturia per mean IPSS Q7 was 2.3 ± 1.2. The mean treatment change was −0.4 with placebo and −0.5 with tadalafil; the least-squares mean (standard error) treatment difference was −0.2 (0.05), p = 0.002. For patients receiving placebo and tadalafil, respectively, the proportion with improved nocturnal frequency was 41.3 and 47.5 %, with no change was 44.8 and 41.0 %, and with worsening was 13.9 and 11.5 %.
Conclusions
A statistically significant improvement in nocturnal frequency was seen with tadalafil over placebo; however, the treatment difference was small and not considered clinically meaningful. Further studies using voiding diaries and excluding patients with nocturnal polyuria would be needed to more precisely estimate the impact of tadalafil on nocturia associated with LUTS/BPH.


Similar content being viewed by others
References
Abrams P, Cardozo L, Fall M et al (2003) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61:37–49
Coyne KS, Zhou Z, Bhattacharyya SK, Thompson CL, Dhawan R, Versi E (2003) The prevalence of nocturia and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int 92:948–954
Tikkinen KA, Johnson TM, Tammela TL et al (2010) Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. Eur Urol 57:488–496
Weiss JP, Blaivas JG, Bliwise DL et al (2011) The evaluation and treatment of nocturia: a consensus statement. BJU Int 108:6–21
Stief CG, Porst H, Neuser D, Beneke M, Ulbrich E (2008) A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol 53:1236–1244
Weiss JP, Blaivas JG (2000) Nocturia. J Urol 163:5–12
Oelke M, Bachmann A, Descazeaud A et al (2013) EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 64:118–140
Kingery L, Martin ML, Naegeli AN, Khan S, Viktrup L (2012) Content validity of the Benign Prostatic Hyperplasia Impact Index (BII); a measure of how urinary trouble and problems associated with BPH may impact the patient. Int J Clin Pract 66:883–890
Roehrborn CG, McVary KT, Elion-Mboussa A, Viktrup L (2008) Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol 180:1228–1234
Porst H, Kim ED, Casabe AR et al (2011) Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of an international randomized, double-blind, placebo-controlled trial. Eur Urol 60:1105–1113
Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L (2012) Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol 61:917–925
Egerdie RB, Auerbach S, Roehrborn CG et al (2012) Tadalafil 2.5 or 5 mg administered once daily for 12 weeks in men with both erectile dysfunction and signs and symptoms of benign prostatic hyperplasia: results of a randomized, placebo-controlled, double-blind study. J Sex Med 9:271–281
Porst H, Oelke M, Goldfischer ER et al (2013) Efficacy and safety of tadalafil 5 mg once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: subgroup analyses of pooled data from 4 multinational, randomized, placebo-controlled clinical studies. Urology 82:667–673
(2010) Cialis [package insert]. http://pi.lilly.com/us/cialis-pi.pdf
(2013) Summary of product characteristics (Cialis). http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000436/WC500026318.pdf
Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, de la Rosette JJ (2004) EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines). Eur Urol 46:547–554
Porst H, Roehrborn CG, Secrest RJ, Esler A, Viktrup L (2013) Effects of tadalafil on lower urinary tract symptoms secondary to benign prostatic hyperplasia and on erectile dysfunction in sexually active men with both conditions: analyses of pooled data from four randomized, placebo-controlled tadalafil clinical studies. J Sex Med 10:2044–2052
Tikkinen KAO, Johnson TM II, Cartwright R (2012) Prevalence and bother of nocturia. In: Oelke M, van Kerrebroeck PEV (eds) Current aspects in diagnosis and treatment of nocturia, 1st edn. UNI-MED-Verlag, Bremen, pp 18–26
Acknowledgments
Thomas Melby (inVentiv Health Clinical, Cary, NC) provided editorial assistance in the preparation of this manuscript. This study was funded by Eli Lilly and Company.
Conflict of interest
MO is a consultant, speaker, or trial investigator for Allergan, Apogepha, Astellas, Ferring, Lilly, Pfizer, Recordati, and Teva. JPW is a consultant for Alleran, Astellas, Ferring, Lilly, Pfizer, and Vantia. CM is a speaker and trial investigator for Lilly. DC, DR, and LV are employees of Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oelke, M., Weiss, J.P., Mamoulakis, C. et al. Effects of tadalafil on nighttime voiding (nocturia) in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a post hoc analysis of pooled data from four randomized, placebo-controlled clinical studies. World J Urol 32, 1127–1132 (2014). https://doi.org/10.1007/s00345-014-1255-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-014-1255-z