-
PDF
- Split View
-
Views
-
Cite
Cite
A. C. Pasqualotto, S. J. Howard, C. B. Moore, D. W. Denning, Flucytosine therapeutic monitoring: 15 years experience from the UK, Journal of Antimicrobial Chemotherapy, Volume 59, Issue 4, April 2007, Pages 791–793, https://doi.org/10.1093/jac/dkl550
Close - Share Icon Share
Abstract
There is uniform consensus that flucytosine blood concentrations should be measured to avoid toxicity and ensure adequate efficacy.
The purpose of this study was to evaluate all flucytosine levels performed in a regional centre in the UK from October 1991 to May 2006. Concentrations were measured by bioassay.
We reviewed 1071 flucytosine levels in 233 patients, including 33 neonates. Overall, only 20.5% of levels were in the expected therapeutic range. Low levels were observed in 40.5%, of which 5.1% were undetectable levels (<12.5 mg/L). High levels occurred in 38.9%, of which 9.9% were considered potentially toxic (>100 mg/L). High flucytosine levels occurred more frequently amongst neonates, which could be related to an immature renal system resulting in drug accumulation.
Our findings reveal that the vast majority of patients were out of range for flucytosine levels. These data emphasize the importance of monitoring flucytosine levels.
Introduction
Flucytosine is a synthetic fluorinated pyrimidine analogue in use since the 1960s. Although one of the oldest antifungal agents in clinical practice, this drug still holds its place as first-line therapy in the treatment of cryptococcal meningoencephalitis, in association with amphotericin B.1 In addition, flucytosine is active against a range of fungal pathogens, including Candida species, Saccharomyces cerevisiae and selected dematiaceous moulds. In combination therapy, flucytosine might be particularly useful where tissue penetration of the other agent is poor, e.g. eye, urine and meninges. However, based on its narrow therapeutic index, blood monitoring has been recommended.2,3 The purpose of this study was to review all flucytosine levels performed in our centre over a 15 year period.
Material and methods
From October 1991 to May 2006 we reviewed all flucytosine levels performed in a regional centre in North-West England.
Flucytosine levels were measured by bioassay. The medium used was yeast nitrogen base (Difco, Surrey, UK) supplemented with 1% glucose and solidified with 2% Oxoid agar no. 1 (Unipath Ltd, Basingstoke, UK). The medium was prepared and seeded with Candida glabrata (strain F/4023, approximately 5 × 107 cfu/mL in 6 mL of sterile distilled H2O). This isolate is resistant to fluconazole and itraconazole (MICs of 128 and > 8 mg/L, respectively), and susceptible to flucytosine (MIC ≤ 0.125 mg/L) and amphotericin B (MIC 0.06 mg/L). Flucytosine standards were prepared by dilution of the 1280 mg/L stock solution in water to give concentrations of 6.25–100 mg/L (2-fold dilution range). Patient samples were diluted 1:2 using sterile distilled H2O to avoid any interference from amphotericin B. Patient samples and standards (30 µL volumes) were applied in a minimum of duplicate in a randomized fashion to 8 mm wells cut into the plate. The plate was allowed to stand for 30 min at room temperature and then incubated at 37°C for 18 h. The inhibition zone diameters were measured with dial calipers and a standard curve was constructed by plotting the mean zone diameter of standards against the log10 drug concentration. Drug concentrations of patient samples were then determined by reference to the standard curve. No change in methodology occurred during the period of study.
Patients were classified as neonates (1–30 days of life) or non-neonates (all others). Pre-dose samplings were those taken just before drug administration (trough levels). Post-dose (peak levels) included either samples taken 30 min after the end of the intravenous infusion or after 2 h for oral therapy.1 Flucytosine target levels (mg/L) were: non-neonates (trough 30–40, peak 70–80);4 neonates (trough 20–40, peak 50–80). All levels > 100 mg/L were considered potentially toxic. Samples with no clear information about timing of sampling were considered random and not analysed. Limits of flucytosine detection were 12.5–200 mg/L.
Descriptive statistics was used to summarize the data. Pearson's χ2 and Fisher's exact test were used to evaluate the association between qualitative variables, and Mann–Whitney test compared quantitative variables, with a bilateral level of significance of 5%. Data analysis was performed with SPSS 11.5.
Results
A total of 1360 levels were available for study. As random levels were excluded (n = 289), 1071 levels were evaluated in 233 patients (33 neonates). The median number of tests per patient was 3.0 (range: 1–32). Samples consisted of serum (96.4%), blood from haemofiltrate (3.4%) and plasma (0.2%). Trough and peak median values were 37.7 mg/L and 66.0 mg/L, respectively.
Overall, only 20.5% of flucytosine levels were in the expected therapeutic range. Levels were low in 40.5% (i.e. trough < 20 mg/L or peak < 50 mg/L for neonates; trough < 30 mg/L or peak < 70 mg/L for non-neonates). Undetectable levels occurred in 5.1%. High levels were observed in 38.9% (i.e. any trough level > 40 mg/L or peak > 80 mg/L), of which 9.9% were potentially toxic levels (>100 mg/L). The persistence of levels in the potentially toxic range for > 2 weeks was documented for only six patients (2.6%).
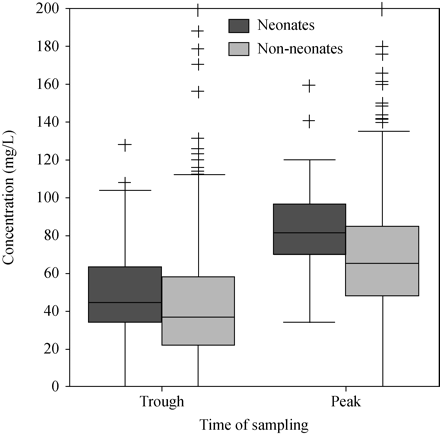
Figure 1 compares the distribution of flucytosine levels for neonates and non-neonates. Neonates had high levels more frequently than non-neonates (60.8% versus 37.3%; P < 0.001). Both trough (P = 0.025) and peak levels (P = 0.006) were higher in neonates than in non-neonates. However, 42.9% of levels from non-neonates were low (neonates 8.1%; P < 0.001). Satisfactory levels mostly came from neonates (31.1% versus 19.8%; P = 0.025).
Distribution of flucytosine levels according to patients' age. Higher levels were observed for neonates. The box plot summarizes the median, quartiles and extreme values for each numeric variable. The box length is the interquartile range. Extreme and outlier values are marked with the ‘plus’ symbol.
Discussion
Therapeutic drug monitoring of flucytosine has been routinely performed in many institutions in the UK. Recently, the group from Bristol described only 17.1% of flucytosine levels obtained from paediatric patients in the expected therapeutic range.4 Similarly, four out of five samples tested in our study were out of range for flucytosine levels. The elevated rate of inappropriate levels observed in these two studies—which together included 1462 samples—suggests that drug monitoring could be of great importance.
In our study, 40.5% of flucytosine levels were below the recommended range. Previous studies have indicated that low flucytosine levels may increase the risk for treatment failure and the emergence of secondary resistance, particularly with levels < 25 mg/L.5 Unfortunately, because of the lack of clinical data we were not able to say whether our patients were on an appropriate flucytosine dosage, were adherent to treatment or suffered from any significant drug interactions.
Flucytosine monitoring may also help to prevent toxicities associated with the use of this drug. Although the mechanism for hepatotoxicity and bone-marrow depression has not been clearly defined, a concentration-dependent risk seems to exist. Liver toxicity can be possibly avoided with careful maintenance of levels < 100 mg/L, and reversible with temporary drug discontinuation or dose reduction. In one study, persistence of levels > 100 mg/L for more than 2 weeks was particularly associated with haematological or hepatic toxicity.6 We believe the most important finding in our study is the observation that 38.9% of flucytosine levels were high, of which 9.9% were potentially toxic concentrations. In a previous investigation involving 30 patients, peak concentrations of flucytosine exceeded 100 mg/L in 48.4%, reinforcing the importance of drug monitoring.7 As in the study from Bristol,4 levels in neonates were higher than in non-neonates. Because flucytosine clearance is proportional to glomerular filtration rate, infants with very low birth weight may accumulate drug leading to high concentrations because of immature renal function. As an important limitation, the proportion of patients with drug toxicity or concomitantly treated with radiation or myelosuppressive drugs was unknown in both studies.
We use bioassay to measure flucytosine levels in our centre. Although no method is universally superior for flucytosine drug monitoring, the bioassay remains the simplest and is a widely used technique.3 Nonetheless, concomitant treatment with other antifungal agents may interfere with flucytosine levels as detected by bioassay. Another limitation of our study was the lack of information about combination antifungal therapy. However, amphotericin B—the main antifungal drug used in combination with flucytosine—does not interfere with flucytosine detection by bioassay, because amphotericin B has limited diffusion. In addition, samples were diluted 1:2 to further minimize any potential contribution. The seed organism (C. glabrata) is resistant to azole drugs, allowing flucytosine levels to be determined in the presence of flucytosine/azole combinations.
It is important to note that, although we use target levels for flucytosine similar to those chosen by other authors,1,2,4,8 these are arbitrary, based mainly on experts' opinion. With the limited data currently available, it is not possible to know the optimal concentrations of flucytosine. Current target peak flucytosine serum concentration is based on patient tolerance and an acceptable incidence of toxicity. This recommendation does not imply that these targets need to be achieved in order to obtain clinical benefit. Nevertheless, therapeutically effective concentrations of flucytosine should exceed the MIC for the pathogen involved. Both the British Society for Medical Mycology (BSMM; formerly British Society for Mycopathology)3 and the Clinical Laboratory and Sciences Institute (CLSI; formerly NCLSS)9 have proposed susceptibility breakpoints for flucytosine. The breakpoints established by the BSMM are: susceptible ≤ 1.0 mg/L, intermediate susceptibility 2.0–8.0 mg/L and resistant ≥16 mg/L.3 In contrast, the CLSI has defined susceptible ≤ 4.0 mg/L, intermediate susceptibility 8.0–16.0 mg/L and resistant ≥32 mg/L. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is yet to publish susceptibility breakpoints for flucytosine. It may be that genotypic markers of resistance more accurately identify non-responsive strains compared with either standard phenotypic method. Our own experimental model work with Candida albicans suggested that much lower concentrations are required for systemic infection with susceptible isolates.10 Further understanding of exposure–response relationships with other organisms is required and may alter current and drug-exposure breakpoints and dosing.
In conclusion, our study revealed that the vast majority (79.5%) of patients were out of range for flucytosine levels. High and potentially toxic levels were observed for 38.9% of patients, of major concern. These data highlight the importance of monitoring flucytosine levels in patients treated with this drug.
Acknowledgements
This study was partially presented in the Clinical Mycology Slide Session at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, USA, 2006 (Abstract M-1305). Dr Pasqualotto receives a grant from CAPES (Brazilian government). The Regional Mycology Laboratory, Manchester has been supported by the Fungal Research Trust for many years.
Transparency declarations
None to declare.