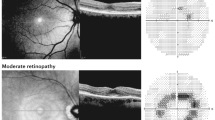
Abstract
Dermatologists use antimalarials to treat conditions such as cutaneous lupus erythematosus. One potentially serious adverse effect of these agents is irreversible maculopathy. Although there is some evidence that hydroxychloroquine and chloroquine have similarly narrow therapeutic indices with regard to retinal toxicity, the former is thought to be less damaging to the retina and is thus more widely employed by dermatologists. The current recommended maximal dose for hydroxychloroquine is 6.5 mg/kg/day, with the weight in kilograms used for this calculation being the ideal bodyweight rather than actual bodyweight. Ophthalmologic follow-up is an important component of monitoring patients taking antimalarials. Recommendations for follow-up frequency have varied, and we present the recent guidelines from the American Academy of Ophthalmology. Despite dose limitations and ophthalmologic monitoring, irreversible retinal damage can occur. Among the reported cases, there does not seem to be any obvious predictor of the development of maculopathy. The idiosyncratic nature of this adverse effect may be related to interindividual differences in drug metabolism. To understand why only some patients develop retinopathy, better pharmacokinetic models need to be developed, and further elucidation of the precise mechanism of retinal damage is required.


Similar content being viewed by others
References
Millard T, Hughes G. Antimalarials. In: Wakelin SH, Maibach HI, editors. Handbook of systemic drug treatment in dermatology. London: Manson Publishing Ltd, 2004: 80–7
Rees RB, Maibach HI. Chloroquine: a review of reactions and dermatologic indications. Arch Dermatol. 1963 Sep; 88: 280–9
Callen JP, Camisa C. Antimalarial agents. In: Wolverton SE, editor. Comprehensive dermatologic drug therapy. Philadelphia (PA): WB Saunders Company, 2001: 251–68
Raines MF, Bhargava SK, Rosen ES. The blood-retinal barrier in chloroquine retinopathy. Invest Ophthalmol Vis Sci. 1989 Aug; 30 (8): 1726–31
Mackenzie AH. Dose refinements in long-term therapy of rheumatoid arthritis with antimalarials. Am J Med. 1983 Jul; 75 (IA): 40–5
American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Guidelines for monitoring drug therapy in rheumatoid arthritis. Arthritis Rheum. 1996 May; 39 (5): 723–31
Physicians’ desk reference. 58th ed. Montvale (NJ): Thomson PDR, 2004
Marmor MF. New American Academy of Ophthalmology recommendations on screening for hydroxychloroquine retinopathy [letter]. Arthritis Rheum. 2003 Jun; 48 (6): 1764
Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002 Jul; 109 (7): 1377–82
Katzung BG. Special aspects of geriatric pharmacology. In: Katzung BG, editor. Basic and clinical pharmacology. 9th ed. New York: McGraw Hill, 2004: 1007–14
Levy GD, Munz SJ, Paschal J, et al. Incidence of hydroxychloroquine retinopathy in 1207 patients in a large multicentre outpatient practice. Arthritis Rheum. 1997 Aug; 40 (8): 1482–6
Bernstein HN. Ocular safety of hydroxychloroquine. Ann Ophthalmol. 1991 Aug; 23 (8): 292–6
Weiner A, Sandberg MA, Gaudio AR, et al. Hydroxychloroquine retinopathy. Am J Ophthalmol. 1991 Nov; 112 (5): 528–34
Falcone PM, Paolini L, Lou PL. Hydroxychloroquine toxicity despite normal dose therapy. Ann Ophthalmol. 1993 Oct; 25 (10): 385–8
Mavrikakis M, Papazoglou S, Sfikakis PP, et al. Retinal toxicity in long term hydroxychloroquine treatment. Ann Rheum Dis. 1996 Mar; 55 (3): 187–9
Bienfang D, Coblyn JS, Liang MH, et al. Hydroxychloroquine retinopathy despite regular ophthalmologic evaluation: a consecutive series. J Rheumatol. 2000 Nov; 27 (11): 2703–6
Warner AE. Early hydroxychloroquine macular toxicity. Arthritis Rheum. 2001 Aug; 44 (8): 1959–61
Alarcon GS. How frequently and how soon should we screen our patients for the presence of antimalarial retinopathy?. [letter] Arthritis Rheum. 2002 Feb; 46 (2): 561
Easterbrook M. Dosage of hydroxychloroquine should be based on ideal body weight: comment on the letter by Alarcon. Arthritis Rheum. 2003 Mar; 48 (3): 863–4
Tell SE, Cutler DJ, Day RO, et al. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol. 1989 Jun; 27 (6): 771–9
Furst DE, Lindsley H, Baethge B, et al. Dose-loading with hydroxychloroquine improves the rate of response in early, active rheumatoid arthritis: a randomized, double-blind six-week trial with eighteen-week extension. Arthritis Rheum. 1999 Feb; 42 (2): 357–65
Tell SE, Cutler DJ, Day RO, et al. A dose-ranging study of the pharmacokinetics of hydroxychloroquine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1988 Sep; 26 (3): 303–13
Tell SE, Cutler DJ, Brown KF. High-performance liquid chromatographic assay for hydroxychloroquine and metabolites in blood and plasma, using a stationary phase of poly(styrene divinylbenzene) and a mobile phase at pH 11, with fluorimetric detection. J Chromatogr. 1985 Nov; 344: 241–8
Tell SE, Cutler DJ, Beck C, et al. Concentration-effect relationship of hydroxychloroquine in patients with rheumatoid arthritis: a prospective, dose ranging study. J Rheumatol. 2000 Jul; 27 (7): 1656–60
Munster T, Gibbs JP, Shen D, et al. Hydroxychloroquine concentration-esponse relationships in patients with rheumatoid arthritis. Arthritis Rheum. 2002 Jun; 46 (6): 1460–9
Carmichael SJ, Charles B, Tell SE. Population pharmacokinetics of hydroxychloroquine in patients with rheumatoid arthritis. Ther Drug Monit. 2003 Dec; 25 (6): 671–81
Shroyer NF, Lewis RA, Lupski JR. Analysis of the ABCR (ABCA4) gene in 4-aminoquinoline retinopathy: is retinal toxicity by chloroquine and hydroxychloroquine related to Stargardt disease?. Am J Ophthalmol. 2001 Jun; 131 (6): 761–6
Acknowledgments
There were no sources of funding and no conflicts of interest directly relevant to the contents of this manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tripp, J.M., Maibach, H.I. Hydroxychloroquine-Induced Retinopathy. Am J Clin Dermatol 7, 171–175 (2006). https://doi.org/10.2165/00128071-200607030-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00128071-200607030-00003