Abstract
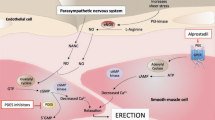
IC351 (tadalafil, trade name Cialis) is a new representative compound of the second generation of selective phosphodiesterase 5 (PDE-5) inhibitors. The selectivity ratio vs PDE-5 is more than 10 000 for PDE-1 through PDE-4 and PDE-7 through PDE-10 and 780 for PDE-6. In the European daily-dosing trial, the efficacy rates were up to 93% for successful intercourses with completion in the 50-mg dose in patients with mild to moderate erectile dysfunction (ED). In two different dose-ranging studies with 2–25 mg taken as needed, efficacy rates of up to 88% improvement in erections and up to 73% successful intercourses with completion were achieved. In a placebo-controlled, fixed-dose (10- and 20-mg) trial in diabetic patients, improved erections of 56% and 64% were reported compared with 25% after placebo. Drug-related adverse effects, with headache in up to 23% of patients (placebo, up to 17%), dyspepsia in up to 11% (placebo, up to 7%), back pain in up to 4.7% (placebo, 0%), and myalgia in up to 4.1% (placebo, up to 2.4%), were mostly mild to moderate. Neither drug-related serious cardiovascular adverse events nor color vision disturbances were encountered. The long half-life (>17 h), with a comfortably long window of opportunity, releases couples from the need to plan sexual activities and therefore provides the highest amount of spontaneity for sexual activities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Porst, H. IC351 (tadalafil, Cialis): update on clinical experience. Int J Impot Res 14 (Suppl 1), S57–S64 (2002). https://doi.org/10.1038/sj.ijir.3900807
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3900807
Keywords
This article is cited by
-
Acute pancreatitis induced by Tadalafil: a case report
Clinical Journal of Gastroenterology (2020)
-
Nebenwirkungen der medikamentösen Behandlung der erektilen Dysfunktion
Der Urologe (2017)
-
Tadalafil
Drugs & Aging (2012)
-
Sexualität der Frau nach onkologischer Therapie
Forum (2012)
-
Do vardenafil and tadalafil have advantages over sildenafil in the treatment of erectile dysfunction?
International Journal of Impotence Research (2007)