Seasonal Variation in Resource Overlap Between Red Swamp Crayfish (Procambarus clarkii) and Native Species in Poyang Lake Wetland, China
- 1College of Life and Environmental Sciences, Hangzhou Normal University, Hangzhou, China
- 2National Ecosystem Research Station of Jiangxi Poyang Lake Wetland, Jiangxi Province Key Laboratory of Watershed Ecosystem Change and Biodiversity, Institute of Life Science, Nanchang University, Nanchang, China
- 3Department of Ecology and Conservation Biology, Texas A&M University, College Station, TX, United States
- 4State Environmental Protection Key Laboratory of Drinking Water Source Management and Technology, Shenzhen Research Academy of Environmental Sciences, Shenzhen, China
- 5Shenzhen Environmental Monitoring Center, Shenzhen, China
Biological invasions are a significant component of current global environmental change that affect biodiversity as well as ecosystem processes and services. The red swamp crayfish (Procambarus clarkii) is one of the most invasive species worldwide, with a documented ability to deplete basal food resources and alter the structure of aquatic food webs. The red swamp crayfish has extensively invaded the Poyang Lake wetland, located in the middle reach of the Yangtze River basin. Here, we use an isotopic mixing model (MixSIAR) with data from stable isotope ratios (δ13C, δ15N) to estimate relative contributions of potential resources to the biomass of red swamp crayfish and ten common native species, and we use hierarchical clustering analysis to assess basal resource breadth and interspecific similarity of invasive and native species. We hypothesized that red swamp crayfish and several native species have similar trophic niches and may compete for basal resources. Results from the mixing model demonstrated seasonal variation in the basal resource of all species, including the red swamp crayfish and native snails, prawns, and fishes. Submerged macrophytes and detritus were estimated to be the most important sources during the rising-water season; during the high-water season, emergent macrophytes and detritus were most important; and during the falling-water season, detritus, POM, and floating macrophytes were most important. Resource overlap was substantial between the invasive crayfish and dominant native species, particularly the freshwater snail (Bellamya aeruginosa), indicating the potential exists for negative impact from competition under conditions of resource limitation.
Introduction
Biological invasions are a significant component of current global environmental change (Sakai et al., 2001; Jackson et al., 2012), with potential to alter biodiversity, ecosystems processes, and human health and welfare (Dudgeon et al., 2006; Chucholl and Chucholl, 2021). For example, biological invasions can impact crop production (Antonio Arce and Dieguez-Uribeondo, 2015) and threaten food security (Cornelissen et al., 2019). Some invaders adapt to novel environments rapidly, and once established, compete with native species (Sakai et al., 2001) and alter the structure of species assemblages (Brenneis et al., 2011; Wang Y. et al., 2021b). Consequently, invasive species are a major cause of biodiversity loss and biotic homogenization (Petsch et al., 2021), acting through mechanisms such as predation, hybridization, and competition (Siesa et al., 2014; Antonio Arce and Dieguez-Uribeondo, 2015). Freshwater systems have experienced extensive biological invasions that impact native biodiversity and important ecosystem services, such as water purification and production of harvestable fish biomass (Dudgeon et al., 2006; Strayer, 2010).
Here we examine the trophic ecology of the invasive red swamp crayfish, Procambarus clarkii (Girard, 1852), a decapod crustacean native to the southern United States and northern Mexico (Hobbs et al., 1989; Larson et al., 2016) that has invaded freshwater habitats in China’s Yangtze River Basin. The red swamp crayfish tolerates extreme environmental conditions (Alcorlo et al., 2004; Anastacio et al., 2009) and has invaded aquatic ecosystems worldwide, now being present on all continents except Antarctica and Australia (Hobbs et al., 1989; Lodge et al., 2012). The crayfish has high fecundity and, following introduction into a new area, can rapidly attain high densities (Correia, 2002) and deplete food resources (Nystrom et al., 2001). Consequently, this invader has the potential to alter food webs and ecosystem processes (Larson et al., 2016). Current knowledge regarding the trophic ecology of the red swamp crayfish mostly pertains to food selection (Alcorlo et al., 2004) and dietary overlap with other crayfish species (Jackson et al., 2014; Larson et al., 2016; Chucholl and Chucholl, 2021).
In China, the red swamp crayfish is now abundant in the Poyang Lake wetlands with nearly half of the prawn catch in 2013 (Zhang et al., 2014), and with approximately 113,000 tones harvested from Dongting Lake in 2017 (Zhang et al., 2020). Both of them indicate the population of red swamp crayfish is abundant in natural habitats. A 10-year ban on fishing within key areas of the Yangtze River was put into effect on 1 January 2020 (Mei et al., 2020). Population density of red swamp crayfish is predicted to increase rapidly following implementation of the fishing ban (Jin et al., 2022). Ecological consequences from this are uncertain, and research is needed to determine options for control of this invasive species in the absence of commercial harvest.
Stable isotope analysis (SIA) can be used to estimate material and energy flow as well as trophic positions in food webs (Peterson and Fry, 1987). SIA has been used increasingly to estimate resource utilization and trophic interaction among sympatric consumers (García et al., 2020; Ercoli et al., 2021; Wang Y. et al., 2021b). The distribution of consumers in isotopic space has been used as an indicator of trophic niche width and trophic niche overlap (Peel et al., 2019; Azevedo et al., 2022). Previous studies have shown that invasive species had higher trophic plasticity (Correia, 2002; Jackson et al., 2016) and greater ability to exploit local resources than native species (Sakai et al., 2001; Britton et al., 2019). Therefore, evaluating the similarity of diet assimilation between invasive and native species can provide insight into the impacts of biological invasion, especially when seasonal variation in resource availability facilitates inferences about interspecific competition (Wang Y. et al., 2021b).
In this study, we used Bayesian mixing models based on δ13C and δ15N to estimated source contributions to consumer tissue, calculated species isotopic spaces and evaluated trophic overlap between the red swamp crayfish and ten dominant native species from the Poyang Lake wetlands during three hydrological seasons (rising water, high water, falling water). Our objectives were to use this information to infer 1) seasonal variation in basal resources, 2) trophic niche width, and 3) trophic niche similarity between the invasive crayfish and native species. We hypothesized that the red swamp crayfish has a similar trophic niche and outcompetes several native species during seasons of resource scarcity. Findings from this study advance understanding of mechanisms by which red swamp crayfish impact native species and support ecosystem management and biological conservation.
Materials and Methods
Study Area
Poyang Lake (28°24′ ∼ 29°46′N, 115°49′ ∼ 116°46′E) is located in the northern part of Jiangxi Province, China, on the south bank of the middle reach of the Yangtze River (Sun et al., 2017). Poyang Lake receives water from five main rivers (Ganjiang, Xiushui, Raohe, Fuhe, Xinjiang) in Jiangxi Province, and has an outlet to the Yangtze River, and which it discharges and receives water depending on river and lake levels (Zhang et al., 2013). The annual runoff from Poyang Lake is around 1.46 × 1011 m3, accounting for about 15.6% of the annual average discharge of the Yangtze River (Wu et al., 2017). Regional climate is subtropical monsoonal (Fang et al., 2018; Jin et al., 2019) and the lake water level ranges from 8 to 22 m, which is associated with major changes in lake surface area (>4,000 km2 during the flood season; <1,000 km2 during the dry season; (Li et al., 2018).
Poyang Lake and associated wetlands comprise a dynamic system that provides important ecosystem services (Chen and Xu, 2021). During the low-water period, the landscape consists of the main lake and hundreds of sub-lakes with dense vegetation that support high biodiversity (Chen et al., 2021). The Poyang Lake wetland is impacted not only by the invasive red swamp crayfish, but also water diversion, pollution, and overfishing that have led to the recent ban imposed by the federal government (Mei et al., 2020).
Sample Collection
Samples were collected during three hydrological seasons: rising water (May 2019), high water (July 2019) and falling water (October 2019) (Wu et al., 2022). The sampling habitats were open water of sub-lakes and seasonally connected river channels in Poyang Lake wetland (Figure 1). The red swamp crayfish (Procambarus clarkii), two native river prawns (Macrobrachium nipponense, Exopalaemon modestus) and two freshwater snails (Bellamya aeruginosa, Cipangopaludina cathayensis) were collected using trap nets, each with 20 compartments (3 m × 0.2 m × 0.14 m) covered by a mesh of 1 cm × 1 cm. At each survey location, trap nets were placed on the substrate at a depth of ∼5 m. Fish were collected using the same trap nets plus experimental gillnets (3 panels, each measuring 50 m × 1.4 m) with stretched mesh sizes of 3, 8, 15 cm.
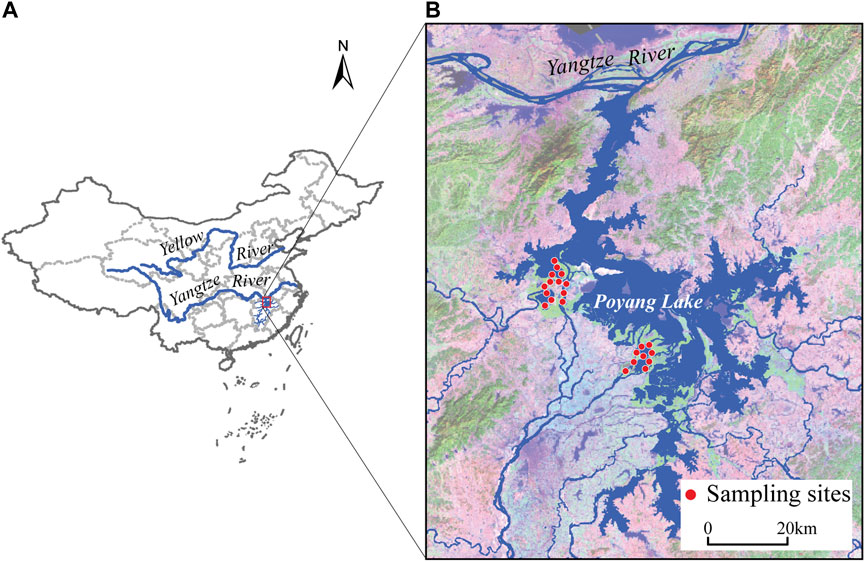
FIGURE 1. Location of Poyang Lake in the Yangtze River Basin, China (A), and locations of sampling sites (B).
Emergent macrophytes (Phragmites australis, Triarrhena lutarioriparia, Zizania latifolia) and floating-leaved macrophytes (Trapa japonica, Nymphoides peltatum) were collected by hand, and submerged macrophytes (Hydrilla verticillata, Vallisneria natans, Ceratophyllum demersum, Potamogeton malaianus, Najas minor) were collected by using a rake (1–3 m long). Samples of suspended particulate organic matter (POM) were collected by filtering water from the water column through Whatman GF/F glass microfiber filters (pore size = 0.7 μM; diameter = 47 mm) aided by a hand-operated vacuum pump. Before use, filters were pre-combusted at 450°C for 12 h. Samples of sediment organic matter (SOM) was collected using a Peterson bottom sampler. Periphyton samples were collected by gently scraping fixed buoys or submerged tree branches with a small spatula. Samples of decaying twigs, leaf litter and other forms of detritus were collected by using a dip net.
While in the field, samples were placed in plastic bags, labeled, and stored on ice, then transferred to the lab and stored in a freezer at −20°C until processing. The carapace was removed from crayfish and shrimps, and a sample of muscle tissue was obtained for stable isotope analysis. For specimens of the six most abundant fish species (index of relative importance, IRI > 500; i.e., Carassius auratus, Cyprinus carpio, Pseudorasbora parva, Tachysurus fulvidraco, Micropercops swinhonis and Rhinogobius giurinus), muscle tissue was removed from the flank near the base of the dorsal fin. For snail samples, muscle tissue was obtained from the foot of snails using dissecting scissors (Chen et al., 2018; Arantes et al., 2019). Samples were rinsed with distilled water before being dried in an oven at 55°C for 48 h, and then ground into a fine powder using mortar and pestle. Finally, each powdered sample was passed through a clean sieve (80 μM mesh) and placed in a tube (2 ml) and stored in a desiccator until shipped for stable isotope analysis.
Stable Isotopic Analysis
Samples were analyzed for content and stable isotope ratios of carbon and nitrogen using an element-isotope ratio mass spectrometer (EA-IRMS; SerCon Integra 2, England) in the Geochemistry and Isotope Laboratory of Third Institute of Oceanography, Ministry of Natural Resources, China. Isotope values were expressed in delta notation according to the formula: δX = [(R sample/R standard)—1] ×103, where X is 13C or 15N, R is the ratio of the heavy to the light isotope like 13C/12C or 15N/14N. The value of δ13C and δ15N were reported as parts per thousand (‰), and the standard material for δ13C was the Vienna Pee Dee Belemnite (VPDB), and for δ15N it was atmospheric N2.
Analytical precision was ± 0.2‰ for both δ13C and δ15N. When the C:N ratio of the consumer tissue sample was <3.5, no lipid correction was done; otherwise, consumer samples were corrected according to the equation: δ13Cnormalized = δ13Cuntreated −3.32 + 0.99 × C:N. δ13Cnormalized was used for subsequent analysis, rather than the δ13Cuntreated (Post et al., 2007).
Data Analyses
All statistical analyses were performed with R software (version 4.1.2; R Core Team, 2021). We used two-way analysis of variance (two-way ANOVA) to test the significance of regional and seasonal differences in δ13C and δ15N of basal resources and consumers. When δ13C or δ15N was significantly different between seasons, we performed Tukey’s multiple comparison test. Prior to performing ANOVA, stable isotope data were log (x+1) transformed to meet assumptions of normality and heteroscedasticity.
We applied a Bayesian Mixing Model (R package MixSIAR) (Stock and Semmens, 2016; Stock et al., 2018) to estimate the relative contributions of alternative sources to consumer tissues during the three hydrological seasons (Stock et al., 2018). We used a trophic discrimination factor of 0.75 ± 0.11‰ for δ13C and 2.75 ± 0.10‰ for δ15N as estimated based on a review of studies of freshwater food webs (Caut et al., 2009). We set parameters for Markov Chain Monte Carlo (MCMC) in the JAGS model in MixSIAR, which included three chains, 30,000 iterations for chain length, burn-in at 200,000 and thin at 100 (Stock et al., 2018; Peel et al., 2019). We set consumer species as a fixed effect, and error structure include residual and process in this model. MCMC generates posterior distributions for estimates of proportional contributions of alternative sources. Results for source contributions included mean, standard deviation, and Bayesian credible intervals. MCMC had two diagnostic test to check convergent performance—the Gelman-Rubin diagnostic and Geweke diagnostic. Values < 1.05 were interpreted to have good mean convergence, otherwise, we repeated the analysis using a longer chain length (McCauley et al., 2012).
To assess similarities in basal resources to the diet assimilation of native and invasive species, one-way hierarchical clustering analysis was performed using the R package flexclust (Leisch, 2006) and data for the relative contributions of potential sources to consumer tissues. The dist function was used to calculate Euclidean distance and average linage was the method selected in the hclust function. Clustering results based on source relative contributions allow inference of trophic similarity between native species and the red swamp crayfish.
To evaluate trophic structure within local communities, six metrics (Layman et al., 2007) and the sample-size-corrected standard ellipse area (SEAc) (Jackson et al., 2011) were calculated using communityMetricsML and groupMetricsML functions in the R package SIBER, respectively (Jackson et al., 2011). Four of these metrics provide information about community trophic diversity, and two are indicators for trophic redundancy. These metrics calculate Euclidean distance among community members within δ13C–δ15N space. Carbon range (CR) provides information about the breadth of resource utilization (Jackson et al., 2012). Nitrogen range (NR) indicates maximum food chain length (Abrantes et al., 2014). Total area (TA) is the area of the convex hull encompassing all individuals in the community and provides a measure of general trophic diversity or niche space. Mean distance to centroid (CD) indicates the average degree of trophic diversity. Mean nearest neighbor distance (MNND) indicates the degree of species packing, with lower values of MNND interpreted as higher trophic redundancy (i.e., more species with similar trophic niches). Standard deviation of nearest neighbor distance (SDNND) estimates the degree of species evenness, with lower values associated with greater trophic redundancy. Because TA is susceptible to the influence of extreme values, SEAc was proposed to indicate core niche width in order to avoid this influence. SEAc was calculated from the variance and covariance of δ13C and δ15N and encompassed approximately 40% of the data (Jackson et al., 2011; Jackson et al., 2012; Britton et al., 2019).
To investigate trophic niche overlap (i.e., potential for competition) among species, we used the bayesianOverlap function in the R package SIBER (Jackson et al., 2011) to calculate the % ellipses overlap between species or groups (SEAc.) SEAc values vary from 0, indicating no overlap, to 1, indicating complete overlap. Values > 0.6 imply significant resource overlap with potential for resource competition (Marufu et al., 2018).
Results
Temporal and Spatial Variation in Isotopic Ratios
Samples from 42 crayfish, 102 snail, 102 prawn and 252 fish specimens were obtained over three hydrological seasons and analyzed for stable isotopes (Table 1). δ13C values of consumers varied significantly among months, with the only exceptions being prawns (M. nipponense, E. modestus) and two fish species (C. carpio, T. fulvidraco). δ13C varied significantly between locations for all species except the snail C. cathayensis. δ15N values of the red swamp crayfish varied significantly among seasons but did not differ between locations. δ15N values of B. aeruginosa, M. nipponense, T. fulvidraco, and R. giurinus varied significantly between seasons and locations (Table 2). Basal resources differed significantly in δ13C values among seasons (no emergent macrophytes were sampled in May 2019), and only POM and SOM had the δ13C values that differed significantly between locations (Table 2). δ15N values of periphyton and submerged macrophytes were the only sources that varied significantly between locations. Moreover, δ15N values of POM, detritus, periphyton and submerged macrophytes, each month were significant difference (Table 2).

TABLE 1. Mean (± 1 SD) values of stable isotope carbon (δ13C) and nitrogen (δ15N) for crayfish, snails, prawns, fishes and basal resources sampled in May 2019 (rising water), July 2019 (high water) and October 2019 (falling water) from Poyang Lake wetland. Samples are from catches of gillnets and trap nets; only common fish species (IRI > 500) were analyzed. POM = suspended particular organic matter, SOM = organic matter in surface sediments.
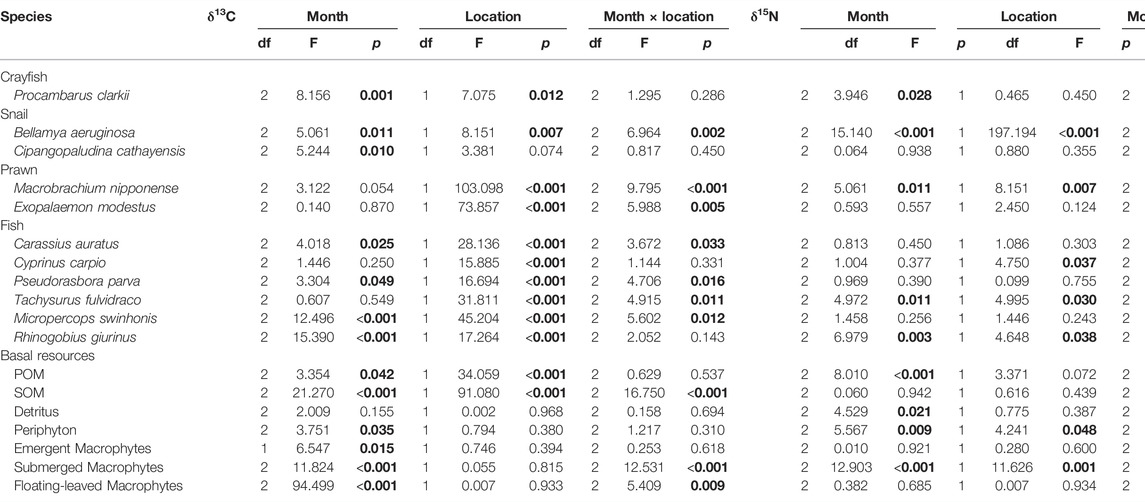
TABLE 2. Results from two-way analysis of variance (two-way ANOVA) testing for effects of month and location on δ13C and δ15N, bold font denotes statistically significant values (p < 0.05). POM = suspended particular organic matter, SOM = organic matter in surface sediments.
δ13C of P. clarkii, C. cathayensis, R. giurinus were significantly higher in July than in May and October. B. aeruginosa δ13C increased significantly from May to October. δ15N signatures of P. clarkii and R. giurinus were significantly higher during July than October (Supplementary Figure S1). δ15N values for B. aeruginosa, M. nipponense and T. fulvidraco were significantly greater during May than October (Supplementary Figure S1).
δ13C and δ15N signatures of P. clarkii were similar to those of snail species, with δ15N close to values of basal resources. Prawns and fish species had similar δ13C and δ15N signatures across all seasons (Figure 2).
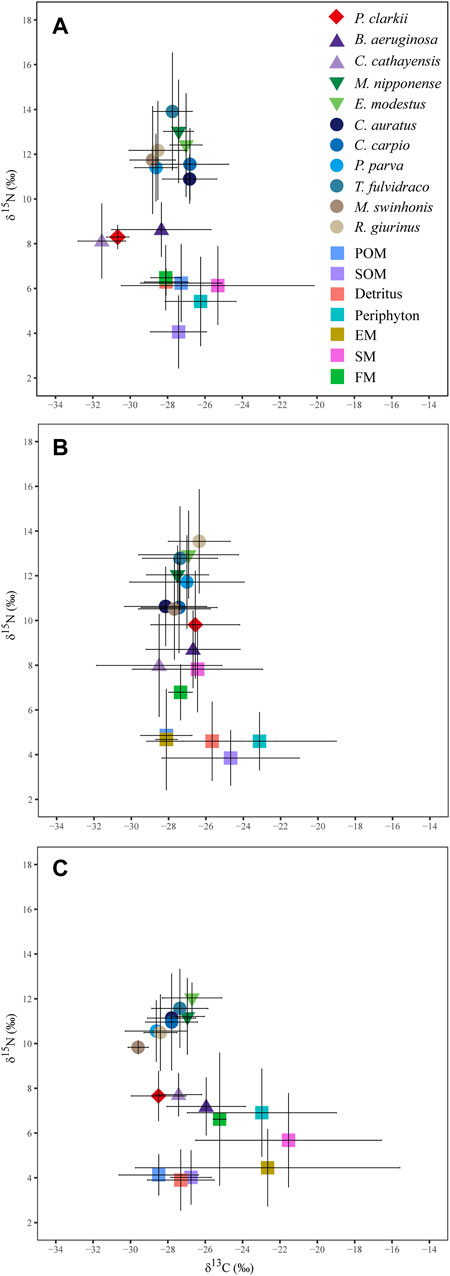
FIGURE 2. Mean (± 1 SD) values of δ13C and δ15N of the red swamp crayfish (diamonds), snail species (inverted triangles), prawn species (upright triangles), fish species (circles) and their potential food sources (squares) from Poyang Lake in May (A), July (B) and October (C) 2019. POM = suspended particular organic matter, SOM = organic matter in surface sediments, EM = emergent macrophytes, SM = submerged macrophytes, FM = floating macrophytes.
Relative Contribution of Basal Resources to the Diet Assimilation of Consumers
MixSIAR model estimates revealed seasonal variation in diet assimilation of nearly all consumers. During the rising-water season, submerged macrophytes were estimated to be the most important source supporting biomass of the red swamp crayfish and snails, whereas prawns relied mostly on detritus (Supplementary Table S1, Supplementary Figure S2). Fishes were supported largely by detritus and submerged macrophytes during the rising-water season (Supplementary Table S1, Supplementary Figure S3). During the high-water season, emergent macrophytes appeared to be the most important source (∼ 43%) supporting the red swamp crayfish, and POM was the second most important resource (∼ 36%). A similar result was obtained for B. aeruginosa, C. cathayensis, C. auratus, C. carpio and M. swinhonis. During the high-water season, M. nipponense, E. modestus, P. parva, T. fulvidraco and R. giurinus assimilated relatively large percentages of material originating from emergent macrophytes, but the second most important source was the floating-leaved macrophytes. During the falling-water season, the estimated contributions from periphyton, emergent macrophytes and submerged macrophytes to consumer biomass were negligible, detritus, POM and floating macrophytes were most important (Supplementary Table S1).
Hierarchical cluster analyses divided consumer taxa into three trophic groups during the rising-water season, four trophic groups during both the high-water and falling-water seasons (Figure 3). During the rising-water season, the red swamp crayfish clustered with snails, and prawns clustered with fishes except C. auratus. During the high-water season, isotopic composition of P. clarkii was similar to that of B. aeruginosa, M. nipponense, C. auratus, C. carpio, T. fulvidraco, M. swinhonis and R. giurinus. Exopalaemon modestus, C. cathayensis and P. parva did not cluster with any of the other taxa. During the falling-water season, taxa clustered largely according to phylogenetic relatedness. P. clarkii shared cluster with B. aeruginosa, and these two taxa were isotopically distinct from C. cathayensis (Figure 3).
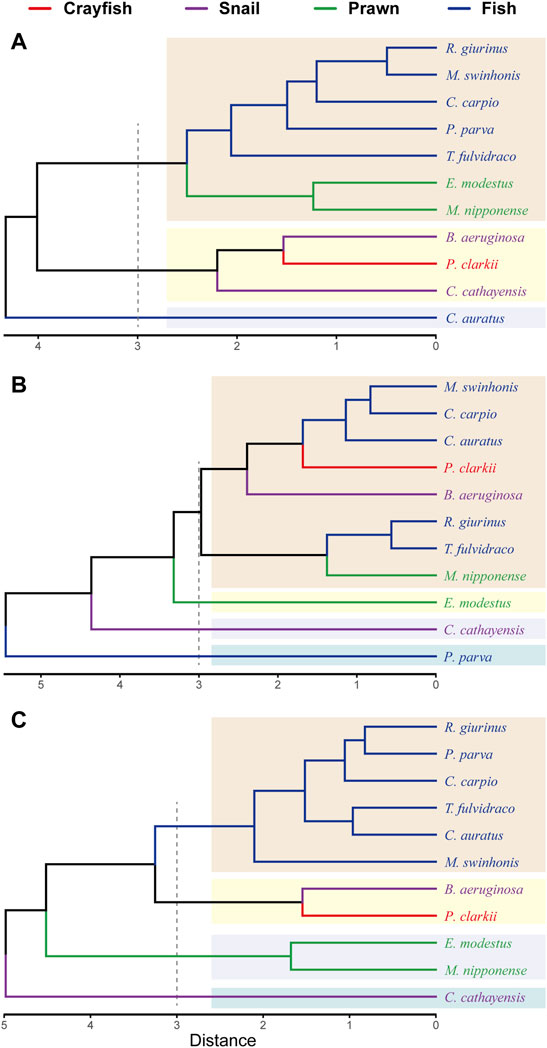
FIGURE 3. Cluster analysis results based on the relative contributions of sources to biomass of red swamp crayfish, snails, prawns and fishes in May (A), July (B) and October (C) 2019. Delimitation of groups was set at a distance of 3.
The isotopic composition of the snail B. aeruginosa was very similar that of the red swamp crayfish during all three periods, but another snail species (C. cathayensis) was dissimilar P. clarkii. Isotopic composition and estimated contributions to biomass of fishes was variable. For example, estimated percent contributions of sources to biomass of C. auratus and P. parva were different from other five fish species during the rising- and high-water seasons.
Trophic Niche Width Among Seasons and Trophic Overlap Between Species
The highest and lowest values of NR, CR, TA, CD, SEAc were recorded during the high- and falling-water seasons, respectively (Table 3), indicating a more greater community trophic diversity during the high-water season when aquatic habitat expands into the floodplain, and lowest trophic diversity when waters gradually drained from the floodplains. Values of MNND and SDNND during the rising-water season were slightly higher when compared to the other seasons (Table 3), suggesting lower density and evenness of species packing when aquatic habitat was expanding into the floodplain.
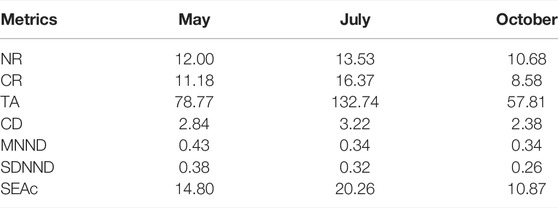
TABLE 3. Metrics for communities comprised of crayfish, snails, prawns and fishes during May, July and October, 2019. NR, nitrogen range; CR, carbon range; TA, total area of convex hull; CD, mean distance to centroid; MNND, mean nearest neighbor distance; SDNND, standard deviation of the nearest neighbor distance; SEAc, the core isotopic niche area of the community (‰2).
During all three periods, fishes tended to cluster with prawns, and crayfish tended to cluster with snails. During the rising-water season, the core isotopic niche area (SEAc) of crayfish was markedly smaller compared its area during other seasons, and the crayfish core area was nested inside the that of snails. During the rising- and falling-water seasons, the SEAc of prawns and fishes were different from those of crayfish and snails, but during the high-water period these groups overlapped extensively (Figure 4).
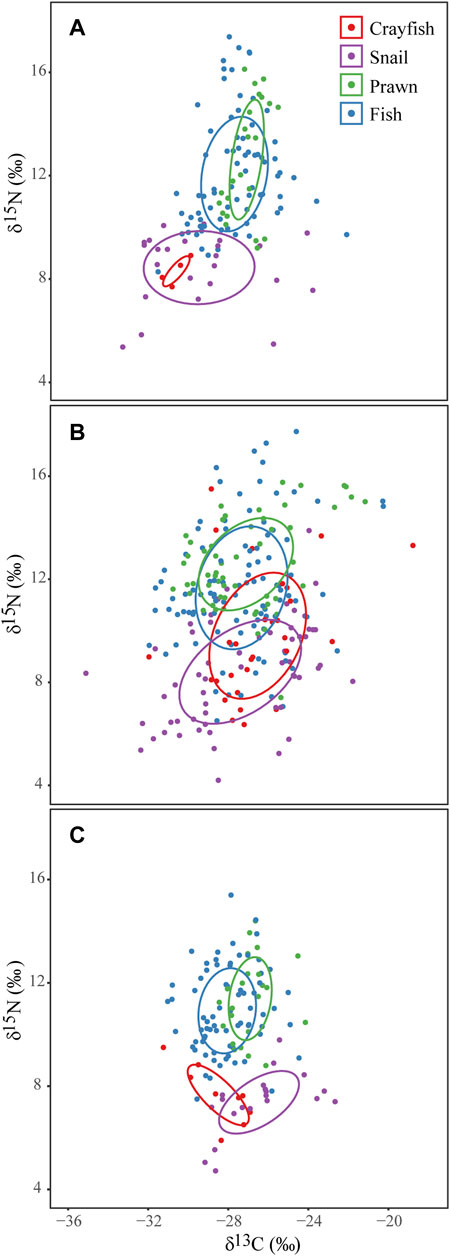
FIGURE 4. Core isotopic niche areas (SEAc) of four groups in Poyang Lake wetland in May (A), July (B) and October (C) 2019.
When SEAc was estimated for all taxa across all three survey periods, the red swamp crayfish had a large SEAc, implying higher trophic diversity compared to other taxa (the only exception was C. cathayensis with SEAc = 18.75). The prawn, M. nipponense, had the narrowest isotopic niche width (SEAc = 7.32), an indication of dietary specialization (Table 4). The red swamp crayfish had large isotopic niche overlap with snails; 61.1% and 59.6% of crayfish area overlapped with B. aeruginosa and C. cathayensis, respectively, and 75.5% of the B. aeruginosa niche and 54.8% of the C. cathayensis area overlapped with the crayfish. In contrast, overlap between the crayfish with prawns was low (9.24% of the isotope niche area of E. modestus was overlapped by P. clarkii, and 7.40% of the area of P. clarkii was overlapped by E. modestus; Table 4). Two thirds of the fish species had SEAc with P. clarkii of approximately 50%, with T. fulvidraco the only fish with low overlap with P. clarkii. There was little or no overlap between snails and prawns, and relatively low overlap between snails and fishes (Table 4, Figure 4).
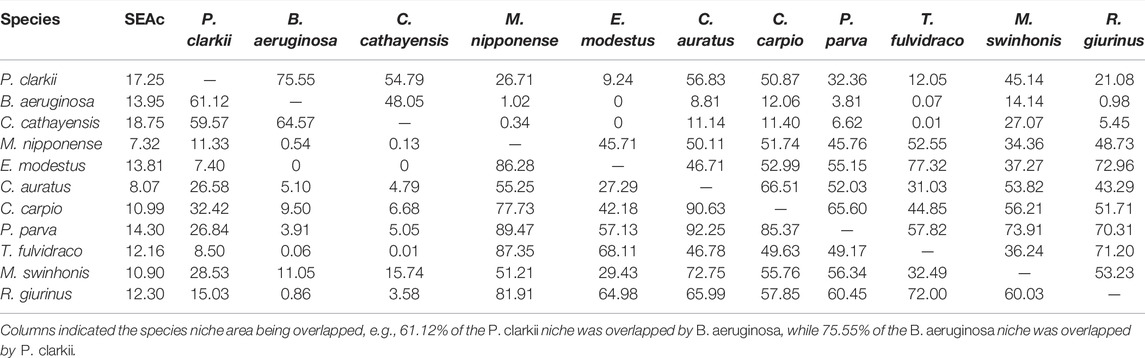
TABLE 4. The core isotopic niche area (SEAc) and percent overlap (%) between species in Poyang Lake wetland in 2019.
Discussion
This study documented seasonal variation in the isotopic composition and estimated contributions of basal resources to biomass of the red swamp crayfish and native snails, prawns, and fishes of the Poyang Lake wetland. Trophic niche diversity of the red swamp crayfish, snails, prawns, and fishes was associated with stages of the annual flood pulse, with greatest diversity observed during the high-water season and lowest diversity during the falling water period, a finding is similar to that reported for the Tonle Sap Lake/wetland system in the lower Mekong Basin (Pool et al., 2017). Highest isotopic overlap, and hence potential for resource competition, was between the crayfish and snails and between fishes and prawns.
Seasonality of Isotopic Signatures
Seasonal variation in the δ13C and δ15N of basal resources and aquatic consumers in the Poyang Lake wetland were likely mediated by temperature, flow, nutrient concentrations, vegetation cover, and other abiotic and biotic environmental factors (Hladyz et al., 2012). Two crayfish species, Orconectes eupunctus and Orconectes neglectus, in a stream in the Ozark Highlands of North America showed significant differences in both δ13C and δ15N between spring and summer (Magoulick and Piercey, 2016).
Higher values of δ13C in basal production sources (e.g., periphyton and macrophytes) can result from greater demands of CO2, which usually happens during the summer. Consumers would then reflect these higher δ13C values if basal sources are being assimilated, either directly or indirectly (Olsson et al., 2008; Guzzo et al., 2011; Hladyz et al., 2012). δ13C values change relatively little as material is transferred between successive trophic levels (Olsson et al., 2008; Brauns et al., 2018), and therefore provides a basis for estimating source contributions to consumer biomass using mixing models (Fry and Sherr, 1984; Peterson and Fry, 1987). δ15N generally increases from 2.5 to 3.4‰ with each trophic level, and therefore provides an index of vertical trophic position (Post, 2002). In our study, δ15N values of the red swamp crayfish and snails were similar across all survey periods, indicating a similar vertical trophic position (Abrantes et al., 2014).
Seasonal Variation in Resource Compositions and Between-Species Similarity
Virtually all consumer taxa revealed significant seasonal variation in isotopic composition and associated estimates for assimilation of material from basal sources. For example, the red swamp crayfish was estimated to have assimilated mostly material from emergent macrophytes and POM during the high-water season, and detritus and POM were estimated to have the largest contributions during the falling-water season. This seasonal variation in source contributions generally derives from changes in the availability and/or quality of alternative food resources (Taylor et al., 2017; Veselý et al., 2020). During rising-water season, expanded aquatic habitat in floodplains with superior environmental conditions promote growth of submerged macrophytes. Deeper water level, reduced light penetration and other conditions during the flood may limit growth of submerged macrophytes (Zhu et al., 2012), and many aquatic macrophytes senesce and decompose during the falling-water season. Seasonal changes in the trophic ecology of fishes in wetland systems also is influenced by ontogenetic diet shifts of aquatic organisms, especially fishes (Akin and Winemiller, 2006). The red swamp crayfish is a trophic generalist (Lang et al., 2020) that includes detritus in its diet (Alcorlo et al., 2004). POM often is the most important basal source in food webs of freshwater and coastal wetlands and is comprised of organic material from a variety of terrestrial and aquatic productions sources (Xu et al., 2019). Our findings corroborate its importance, with POM estimated to make major contributions to consumer biomass during the high- and falling-water seasons.
Hierarchical clustering analysis based on estimated sources contributions to consumer biomass revealed similarity within major taxonomic groups (e.g., fish species tended to cluster, prawns tended to cluster, etc.); however, some distantly related taxa, such as crayfish and snails or prawns and fish, also clustered. The red swamp crayfish clustered tightly with the native freshwater snail B. aeruginosa. Another common snail, C. cathayensis, clustered with B. aeruginosa and the red swamp crayfish during the rising-water period but was dissimilar during other seasons. Although fish species tended to cluster with other fishes, some species were divergent from other fishes, such as C. auratus during the rising-water season and P. parva during the high-water season. Such patterns may reflect trophic niche partitioning (Park et al., 2017).
During the high-water period, we found that one cluster included a snail (B. aeruginosa), a prawn (M. nipponense) and five fish species (C. auratus, C. carpio, T. fulvidraco, M. swinhonis, R. giurinus), indicating resource similarity among them. The seasonal flood pulse stimulates reproduction of aquatic organisms (King et al., 2003; Anastacio et al. 2009; Helms et al., 2013) and populations dominated by juvenile recruits feeding on the same basal resources and microfauna and may result in high trophic similarity among species, even some distantly related taxa. Moreover, the flood pulse provides aquatic organisms with access to abundant and/or nutritious resources that are unavailable during the low-water period (Correa and Winemiller, 2014).
Seasonal Variation in Community Trophic Structure and Species Interactions
Five metrics (i.e., NR, CR, TA, CD, SEAc) were positively associated with flood pulse, with intermediate values during the rising-water season, greatest values during the high-water season, and lowest values during the falling-water season. During the high-water season, food chains were longer, and community trophic diversity was greater. Values of MNND and SDNND tended to decline from rising to high to falling water conditions, which implied that aquatic consumers exploited similar resources during falling water season (i.e., higher trophic redundancy) (Wang S. et al., 2021a). Changes in food availability and diversity may result in differences in CR, an indicator for trophic niche diversity (Azevedo et al., 2022). The flood pulse concept predicts that aquatic organisms (e.g., fish) have access to more diverse habitats and food resources during floodplain inundation (Junk et al., 1989; Pool et al., 2017). During the falling-water season, the range of nitrogen isotopic ratios (NR = 10.68), suggesting a reduction in food chain length (Taylor et al., 2017). Resource availability has been proposed as a major factor in determining food chain length (Wang et al., 2016), and low-water conditions in wetlands limit the access of aquatic organisms to diverse terrestrial resources (Wang et al., 2016). Overall, this study showed evidence for the flooding could promote availability and diversity of food resources for consumers resulting in greater trophic niche width (e.g., TA, CD and SEAc) in flood season.
Overlap among isotopic niches generally was greater during the high-water season, suggesting that floodplain inundation facilitated access and exploitation of high-quality resources (Pool et al., 2017). During rising- and falling-water seasons, crayfish and snails had trophic niches that were distinct from fishes and prawns, suggesting niche partitioning (Jackson et al., 2014). During the falling-water season, overlap between crayfish and snails decreased slightly but remained large.
The larger isotopic trophic niche (i.e., high value of SEAc) of the red swamp crayfish compared to the prawn M. nipponense and goldfish C. auratus suggests the invader exploits a greater variety of food resources (Sakai et al., 2001; Britton et al., 2019). Isotopic niche overlap between the crayfish and the prawns M. nipponense and E. modestus ranged from 7.4% to 26.7%, suggesting niche partitioning, which contrasts with results obtained by previous study (Mao et al., 2016). Their results showed high degrees of dietary overlap between P. clarkii and M. nipponense, ranging from 69.7% to 87.8% based on gut contents analysis and stable isotope analysis (Mao et al., 2016). In Poyang Lake wetland, niche overlap among P. clarkii and the snails C. cathayensis and B. aeruginosa ranged from 54.8% to 75.5%, indicating that there is potential for interspecific competition (Britton et al., 2019) but also that resource availability may exceed demand (Gonzalez et al., 2019). The red swamp crayfish has a broad diet (Anastacio et al., 2009; Veselý et al., 2020), and it can shift its foraging when preferred resources are limiting (Correia, 2002). Success of invasive species has been attributed to behavioral plasticity and generalist feeding (Jackson et al., 2016) in addition to broad environmental tolerance (Alcorlo et al., 2004; Anastacio et al., 2009; Loureiro et al., 2015).
Invasive crayfish can impact populations of native species via multiple mechanisms, such as competition, predation (Lodge et al., 2000; Wood et al., 2017). A long-term study revealed that invasion by the rusty crayfish (Orconectes rusticus), was accompanied by gradual reduction of populations of fish species that had diets similar to that of crayfish, whereas piscivorous fishes showed no decline (Wilson et al., 2004). Crayfish have been shown to prey upon small snails thereby affecting recruitment (Parkyn et al., 1997; Nystrom et al., 2001; Hollows et al., 2002). Some fish species (e.g., black carp, Mylopharyngodon piceus) specialize on snails and bivalves (Froese and Pauly, 2019) and may be negatively impacted by competition from invasive crayfish (James et al., 2015). Other study showed that predation by invasive red swamp crayfish significantly reduced the abundance of native freshwater shrimp (Atyaephyra desmarestii) in the Sorraia River Basin, Portugal (Banha and Anastácio, 2011). Consequently, we unable to ignore potential risks that caused by invasive crayfish. In Yangtze River Basin, past fishing may have kept the red swamp crayfish populations under control, but since the fishing ban, the pressure of crayfish invasion is bound to increase. Therefore, it is necessary to strengthen the ecological prevention and control of the red swamp crayfish. Understanding seasonal variation in resource utilization of the red swamp crayfish and resource overlap between invasive crayfish and native species are the important steps towards to ecosystem management and biological conservation.
Conclusion
Here, we used SIA to study the trophic ecology of the red swamp crayfish and resource overlap between invasive and native species. Findings from our study combined with evidence from research conducted in other regions leads to the conclusion that invasive red swamp crayfish have the potential to cause significant impacts to the native aquatic fauna of wetlands in the Yangtze River Basin. More research is warranted to improve understanding of potential impacts of invasive crayfish on native species and ecosystem dynamics. Combining stomach content analysis, fatty acid analysis and SIA can provide more comprehensive information about the dietary of invasive crayfish and trophic interactions between invasive crayfish and native species. Furthermore, considering the effects of sexual and body size of the red swamp crayfish is good to evaluate the ecological role on native communities. These understandings are essential for effective conservation of aquatic biodiversity and fisheries management in the Yangtze River Basin.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
Conceptualization, methodology, investigation, writing—original draft, writing—review and editing, JW; conceptualization, methodology, supervision, project administration, funding acquisition, writing—review and editing, HC and BJ; conceptualization, methodology, supervision, writing—review and editing, KW; methodology, writing—review and editing, SW, WX, HZ and XW.
Funding
This study was supported by the National Key Research and Development Project (2018YFD0900806 and 2018YFD0900906), Fisheries Resources and Environmental Survey in the Yangtze River (CJDC-2017), the National Nature Science Foundation of China (31960254, 31260107, 31770534), and Texas A&M AgriLife Research and the estate of George and Carolyn Kelso via the International Sportfish Fund.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.923962/full#supplementary-material
References
Abrantes, K. G., Barnett, A., and Bouillon, S. (2014). Stable Isotope-Based Community Metrics as a Tool to Identify Patterns in Food Web Structure in East African Estuaries. Funct. Ecol. 28, 270–282. doi:10.1111/1365-2435.12155
Akin, S., and Winemiller, K. O. (2006). Seasonal Variation in Food Web Composition and Structure in a Temperate Tidal Estuary. Estuar. Coast. 29, 552–567. doi:10.1007/bf02784282
Alcorlo, P., Geiger, W., and Otero, M. (2004). Feeding Preferences and Food Selection of the Red Swamp Crayfish, Procambarus Clarkii, in Habitats Differing in Food Item Diversity. Crustac 77, 435–453. doi:10.1163/1568540041643283
Anastácio, P. M., Leitão, A. S., Boavida, M. J., and Correia, A. M. (2009). Population Dynamics of the Invasive Crayfish (Procambarus Clarkii Girard, 1852) at Two Marshes with Differing Hydroperiods. Ann. Limnol. - Int. J. Lim. 45, 247–256. doi:10.1051/limn/2009025
Antonio Arce, J., and Dieguez-Uribeondo, J. (2015). Structural Damage Caused by the Invasive Crayfish Procambarus Clarkii (Girard, 1852) in Rice Fields of the Iberian Peninsula: A Study Case. Fund. Appl. Limnlog. 186, 259–269. doi:10.1127/fal/2015/0715
Arantes, C. C., Winemiller, K. O., Petrere, M., and Freitas, C. E. C. (2019). Spatial Variation in Aquatic Food Webs in the Amazon River Floodplain. Freshw. Sci. 38, 213–228. doi:10.1086/701841
Azevedo, L. S., Pestana, I. A., Almeida, M. G., Bastos, W. R., and Souza, C. M. M. (2022). Do fish Isotopic Niches Change in an Amazon Floodplain Lake over the Hydrological Regime? Ecol. Freshw. Fish. 31, 72–80. doi:10.1111/eff.12609
Banha, F., and Anastácio, P. M. (2011). Interactions between Invasive Crayfish and Native River Shrimp. Knowl. Managt. Aquat. Ecosyst. 401, 17–28. doi:10.1051/kmae/2011033
Brauns, M., Boëchat, I. G., de Carvalho, A. P. C., Graeber, D., Gücker, B., Mehner, T., et al. (2018). Consumer-Resource Stoichiometry as a Predictor of Trophic Discrimination (Δ13C, Δ15N) in Aquatic Invertebrates. Freshw. Biol. 63, 1240–1249. doi:10.1111/fwb.13129
Brenneis, V. E. F., Sih, A., and de Rivera, C. E. (2011). Integration of an Invasive Consumer into an Estuarine Food Web: Direct and Indirect Effects of the New Zealand Mud Snail. Oecologia 167, 169–179. doi:10.1007/s00442-011-1962-8
Britton, J. R., Gutmann Roberts, C., Amat Trigo, F., Nolan, E. T., and De Santis, V. (2019). Predicting the Ecological Impacts of an Alien Invader: Experimental Approaches Reveal the Trophic Consequences of Competition. J. Anim. Ecol. 88, 1066–1078. doi:10.1111/1365-2656.12996
Caut, S., Angulo, E., and Courchamp, F. (2009). Variation in Discrimination Factors (Δ15N And Δ13C): The Effect of Diet Isotopic Values and Applications for Diet Reconstruction. J. Appl. Ecol. 46, 443–453. doi:10.1111/j.1365-2664.2009.01620.x
Chen, M., and Xu, X. (2021). Lake Poyang Ecosystem Services Changes in the Last 30 Years. J. Lake Sci. 33, 309–318. [in Chinese with English abstract]. doi:10.18307/2021.0126
Chen, J., Yang, H., Zeng, Y., Guo, J., Song, Y., and Ding, W. (2018). Combined Use of Radiocarbon and Stable Carbon Isotope to Constrain the Sources and Cycling of Particulate Organic Carbon in a Large Freshwater Lake, China. Sci. Total Environ. 625, 27–38. doi:10.1016/j.scitotenv.2017.12.275
Chen, J., Li, Y. L., Zhou, J. F., Lu, J. Y., Wei, L., Li, W., et al. (2021). Assessing Surface Water-Groundwater Interactions in the Seasonal Lake-Wetland System of Lake Poyang. J. Lake Sci. 33, 842–853. (in Chinese with English abstract). doi:10.18307/2021.0317
Chucholl, F., and Chucholl, C. (2021). Differences in the Functional Responses of Four Invasive and One Native Crayfish Species Suggest Invader‐Specific Ecological Impacts. Freshw. Biol. 66, 2051–2063. doi:10.1111/fwb.13813
Cornelissen, B., Neumann, P., and Schweiger, O. (2019). Global Warming Promotes Biological Invasion of a Honey Bee Pest. Glob. Change Biol. 25, 3642–3655. doi:10.1111/gcb.14791
Correa, S. B., and Winemiller, K. O. (2014). Niche Partitioning Among Frugivorous Fishes in Response to Fluctuating Resources in the Amazonian Floodplain Forest. Ecology 95, 210–224. doi:10.1890/13-0393.1
Correia, A. M. (2002). Niche Breadth and Trophic Diversity: Feeding Behaviour of the Red Swamp Crayfish (Procambarus Clarkii) towards Environmental Availability of Aquatic Macroinvertebrates in a Rice Field (Portugal). Acta Oecol. 23, 421–429. doi:10.1016/s1146-609x(02)01166-9
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z.-I., Knowler, D. J., Lévêque, C., et al. (2006). Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. 81, 163–182. doi:10.1017/S1464793105006950
Ercoli, F., Ghia, D., Gruppuso, L., Fea, G., Bo, T., and Ruokonen, T. J. (2021). Diet and Trophic Niche of the Invasive Signal Crayfish in the First Invaded Italian Stream Ecosystem. Sci. Rep. 11, 8704. doi:10.1038/s41598-021-88073-2
Fang, G. J., Sun, L. Y., Tang, Y. L., Yang, W. Z., and Wang, J. Q. (2018). A Comparative Study on Fishery Resource of Artificial Reefs Based on Gillnet and Cage Catches. Periodical Ocean Univ. China 48, 23–33. (in Chinese with English abstract). doi:10.16441/j.cnki.hdxb.20170349
Froese, R., and Pauly, D. (2019). FishBase: World Wide Web Electronic Publication. Available at: http://www.fishbase.org (Accessed February, 2022).
Fry, B., and Sherr, E. B. (1984). δ13C Measurements as Indicators of Carbon Flow in Marine and Freshwater Ecosystems. Contrib. Mar. Sci. 27, 13–47. doi:10.1007/978-1-4612-3498-212
García, K., Sanpera, C., Jover, L., Palazón, S., Gosálbez, J., Górski, K., et al. (2020). High Trophic Niche Overlap between a Native and Invasive Mink Does Not Drive Trophic Displacement of the Native Mink during an Invasion Process. Animals 10, 1387–1400. doi:10.3390/ani10081387
Girard, C. F. (1852). A Revision of the North American Astaci, With Observations on Their Habits and Geographical Distribution. Proc. Acad. Nat. Sci. Philadelphia 6, 87–91.
Gonzalez, J. G., Ménard, F., Le Loc'h, F., Andrade, H. A. d., Viana, A. P., Ferreira, V., et al. (2019). Trophic Resource Partitioning of Two Snook Fish Species (Centropomidae) in Tropical Estuaries in Brazil as Evidenced by Stable Isotope Analysis. Estuar. Coast. Shelf Sci. 226, 106287. doi:10.1016/j.ecss.2019.106287
Guzzo, M. M., Haffner, G. D., Sorge, S., Rush, S. A., and Fisk, A. T. (2011). Spatial and Temporal Variabilities of Δ13c And Δ15n Within Lower Trophic Levels of a Large Lake: Implications for Estimating Trophic Relationships of Consumers. Hydrobiologia 675, 41–53. doi:10.1007/s10750-011-0794-1
Helms, B., Budnick, W., Pecora, P., Skipper, J., Kosnicki, E., Feminella, J., et al. (2013). The Influence of Soil Type, Congeneric Cues, and Floodplain Connectivity on the Local Distribution of the Devil Crayfish (Cambarus Diogenes Girard). Freshw. Sci. 32, 1333–1344. doi:10.1899/12-160.1
Hladyz, S., Nielsen, D. L., Suter, P. J., and Krull, E. S. (2012). Temporal Variations in Organic Carbon Utilization by Consumers in a Lowland River. River Res. Applic. 28, 513–528. doi:10.1002/rra.1467
Hobbs, H. H., Jass, J. P., and Huner, J. V. (1989). A Review of Global Crayfish Introductions with Particular Emphasis on Two North American Species (Decapoda, Cambaridae). Crustac 56, 299–316. doi:10.1163/156854089x00275
Hollows, J. W., Townsend, C. R., and Collier, K. J. (2002). Diet of the crayfish Paranephrops Zealandicusin Bush and Pasture Streams: Insights from Stable Isotopes and Stomach analysis. New Zeal. J. Mar. Fresh. 36, 129–142. doi:10.1080/00288330.2002.9517076
Jackson, A. L., Inger, R., Parnell, A. C., and Bearhop, S. (2011). Comparing Isotopic Niche Widths Among and within Communities: SIBER - Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602. doi:10.1111/j.1365-2656.2011.01806.x
Jackson, M. C., Donohue, I., Jackson, A. L., Britton, J. R., Harper, D. M., and Grey, J. (2012). Population-Level Metrics of Trophic Structure Based on Stable Isotopes and Their Application to Invasion Ecology. Plos One 7, e31757. doi:10.1371/journal.pone.0031757
Jackson, M. C., Jones, T., Milligan, M., Sheath, D., Taylor, J., Ellis, A., et al. (2014). Niche Differentiation Among Invasive Crayfish and Their Impacts on Ecosystem Structure and Functioning. Freshw. Biol. 59, 1123–1135. doi:10.1111/fwb.12333
Jackson, M. C., Woodford, D. J., Bellingan, T. A., Weyl, O. L. F., Potgieter, M. J., Rivers‐Moore, N. A., et al. (2016). Trophic Overlap between Fish and Riparian Spiders: Potential Impacts of an Invasive Fish on Terrestrial Consumers. Ecol. Evol. 6, 1745–1752. doi:10.1002/ece3.1893
James, J., Slater, F. M., Vaughan, I. P., Young, K. A., and Cable, J. (2015). Comparing the Ecological Impacts of Native and Invasive Crayfish: Could Native Species' Translocation Do More Harm Than Good? Oecologia 178, 309–316. doi:10.1007/s00442-014-3195-0
Jin, B.-S., Winemiller, K. O., Shao, B., Si, J.-K., Jin, J.-F., and Ge, G. (2019). Fish Assemblage Structure in Relation to Seasonal Environmental Variation in Sub-Lakes of the Poyang Lake Floodplain, China. Fish. Manag. Ecol. 26, 131–140. doi:10.1111/fme.12333
Jin, B., Winemiller, K. O., Ren, W., Tickner, D., Wei, X., Guo, L., et al. (2022). Basin‐Scale Approach Needed for Yangtze River Fisheries Restoration. Fish Fish. doi:10.1111/faf.12657
Junk, W. J., Bayley, P. B., and Sparks, R. E. (1989). The Flood Pulse Concept in River-Floodplain Systems. Can. J. Fish. Aquat. Sci. 106, 110–127.
King, A. J., Humphries, P., and Lake, P. S. (2003). Fish Recruitment on Floodplains: The Roles of Patterns of Flooding and Life History Characteristics. Can. J. Fish. Aquat. Sci. 60, 773–786. doi:10.1139/f03-057
Lang, I., Evangelista, C., Everts, R. M., Loot, G., and Cucherousset, J. (2020). Stable Resource Polymorphism along the Benthic Littoral-Pelagic Axis in an Invasive Crayfish. Ecol. Evol. 10, 2650–2660. doi:10.1002/ece3.6095
Larson, E. R., Twardochleb, L. A., and Olden, J. D. (2016). Comparison of Trophic Function between the Globally Invasive Crayfishes Pacifastacus Leniusculus and Procambarus Clarkii. Limnology 18, 275–286. doi:10.1007/s10201-016-0505-8
Layman, C. A., Arrington, D. A., Montana, C. G., and Post, D. M. (2007). Can Stable Isotope Ratios Provide for Community-Wide Measures of Trophic Structure? Ecology 88, 42–48. doi:10.1890/0012-9658(2007)88[42:csirpf]2.0.co;2
Leisch, F. (2006). A Toolbox for -Centroids Cluster Analysis. Comput. Statistics Data Analysis 51, 526–544. doi:10.1016/j.csda.2005.10.006
Li, Y., Yao, J., Zhao, G., and Zhang, Q. (2018). Evidences of Hydraulic Relationships between Groundwater and Lake Water across the Large Floodplain Wetland of Poyang Lake, China. Water Sci. Tech-W. Sup. 18, 698–712. doi:10.2166/ws.2017.150
Lodge, D. M., Taylor, C. A., Holdich, D. M., and Skurdal, J. (2000). Nonindigenous Crayfishes Threaten North American Freshwater Biodiversity: Lessons from Europe. Fisheries 25, 7–20. doi:10.1577/1548-8446(2000)025<0007:nctnaf>2.0.co;2
Lodge, D. M., Deines, A., Gherardi, F., Yeo, D. C. J., Arcella, T., Baldridge, A. K., et al. (2012). Global Introductions of Crayfishes: Evaluating the Impact of Species Invasions on Ecosystem Services. Annu. Rev. Ecol. Evol. Syst. 43, 449–472. doi:10.1146/annurev-ecolsys-111511-103919
Loureiro, T. G., Anastácio, P. M. S. G., Araujo, P. B., Souty-Grosset, C., and Almerão, M. P. (2015). Red Swamp Crayfish: Biology, Ecology and Invasion - An Overview. Nauplius 23, 1–19. doi:10.1590/s0104-64972014002214
Magoulick, D. D., and Piercey, G. L. (2016). Trophic Overlap between Native and Invasive Stream Crayfish. Hydrobiologia 766, 237–246. doi:10.1007/s10750-015-2457-0
Mao, Z., Gu, X., and Zeng, Q. (2016). Food Sources and Trophic Relationships of Three Decapod Crustaceans: Insights from Gut Contents and Stable Isotope Analyses. Aquac. Res. 47, 2888–2898. doi:10.1111/are.12739
Marufu, L., Dalu, T., Crispen, P., Barson, M., Simango, R., Utete, B., et al. (2018). The Diet of an Invasive Crayfish, Cherax Quadricarinatus (Von Martens, 1868), in Lake Kariba, Inferred Using Stomach Content and Stable Isotope Analyses. Bir 7, 121–132. doi:10.3391/bir.2018.7.2.03
McCauley, D. J., Young, H. S., Dunbar, R. B., Estes, J. A., Semmens, B. X., and Micheli, F. (2012). Assessing the Effects of Large Mobile Predators on Ecosystem Connectivity. Ecol. Appl. 22, 1711–1717. doi:10.1890/11-1653.1
Mei, Z., Cheng, P., Wang, K., Wei, Q., Barlow, J., and Wang, D. (2020). A First Step for the Yangtze. Science 367, 1314. doi:10.1126/science.abb5537
Nystrom, P., Svensson, O., Lardner, B., Bronmark, C., and Graneli, W. (2001). The Influence of Multiple Introduced Predators on a Littoral Pond Community. Ecology 82, 1023–1039. doi:10.2307/2679900
Olsson, K., Nyström, P., Stenroth, P., Nilsson, E., Svensson, M., and Granéli, W. (2008). The Influence of Food Quality and Availability on Trophic Position, Carbon Signature, and Growth Rate of an Omnivorous Crayfish. Can. J. Fish. Aquat. Sci. 65, 2293–2304. doi:10.1139/f08-137
Park, J. M., Gaston, T. F., and Williamson, J. E. (2017). Resource Partitioning in Gurnard Species Using Trophic Analyses: The Importance of Temporal Resolution. Fish. Res. 186, 301–310. doi:10.1016/j.fishres.2016.10.005
Parkyn, S. M., Rabeni, C. F., and Collier, K. J. (1997). Effects of Crayfish (Paranephrops Planifrons: Parastacidae) on In‐Stream Processes and Benthic Faunas: A Density Manipulation Experiment. N. Z. J. Mar. Freshw. Res. 31, 685–692. doi:10.1080/00288330.1997.9516798
Peel, R. A., Hill, J. M., Taylor, G. C., and Weyl, O. L. F. (2019). Food Web Structure and Trophic Dynamics of a Fish Community in an Ephemeral Floodplain Lake. Front. Environ. Sci. 7, 1–18. doi:10.3389/fenvs.2019.00192
Peterson, B. J., and Fry, B. (1987). Stable Isotopes in Ecosystem Studies. Annu. Rev. Ecol. Syst. 18, 293–320. doi:10.1146/annurev.es.18.110187.001453
Petsch, D. K., Ribas, L. G. D. S., Mantovano, T., Pulzatto, M. M., Alves, A. T., Pinha, G. D., et al. (2021). Invasive Potential of Golden and Zebra Mussels in Present and Future Climatic Scenarios in the New World. Hydrobiologia 848, 2319–2330. doi:10.1007/s10750-020-04412-w
Pool, T., Holtgrieve, G., Elliott, V., McCann, K., McMeans, B., Rooney, N., et al. (2017). Seasonal Increases in Fish Trophic Niche Plasticity within a Flood-Pulse River Ecosystem (Tonle Sap Lake, Cambodia). Ecosphere 8, e01881–15. doi:10.1002/ecs2.1881
Post, D. M., Layman, C. A., Arrington, D. A., Takimoto, G., Quattrochi, J., and Montaña, C. G. (2007). Getting to the Fat of the Matter: Models, Methods and Assumptions for Dealing with Lipids in Stable Isotope Analyses. Oecologia 152, 179–189. doi:10.1007/s00442-006-0630-x
Post, D. M. (2002). Using Stable Isotopes to Estimate Trophic Position: Models, Methods, and Assumptions. Ecology 83, 703–718. doi:10.2307/3071875
R Core Team (2021). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available at: http://www.R-project.org/.
Sakai, A. K., Allendorf, F. W., Holt, J. S., Lodge, D. M., Molofsky, J., With, K. A., et al. (2001). The Population Biology of Invasive Species. Annu. Rev. Ecol. Syst. 32, 305–332. doi:10.1146/annurev.ecolsys.32.081501.114037
Siesa, M. E., Padoa-Schioppa, E., Ott, J., De Bernardi, F., and Ficetola, G. F. (2014). Assessing the Consequences of Biological Invasions on Species with Complex Life Cycles: Impact of the Alien Crayfish Procambarus Clarkii on Odonata. Ecol. Indic. 46, 70–77. doi:10.1016/j.ecolind.2014.05.036
Strayer, D. L., and Semmens, B. X. (2016). MixSIAR GUI User Manual. Version 3.1. Available at: https://github.com/brianstock/MixSIAR. doi:10.5281/zenodo.1209993
Stock, B. C., Jackson, A. L., Ward, E. J., Parnell, A. C., Phillips, D. L., and Semmens, B. X. (2018). Analyzing Mixing Systems Using a New Generation of Bayesian Tracer Mixing Models. PeerJ 6, e5096. doi:10.7717/peerj.5096
Strayer, D. L. (2010). Alien Species in Fresh Waters: Ecological Effects, Interactions with Other Stressors, and Prospects for the Future. Freshwater. Biol. 55, 152–174. doi:10.1111/j.1365-2427.2009.02380.x
Sun, C., Zhen, L., and Giashuddin Miah, M. (2017). Comparison of the Ecosystem Services provided by China's Poyang Lake Wetland and Bangladesh's Tanguar Haor Wetland. Ecosyst. Serv. 26, 411–421. doi:10.1016/j.ecoser.2017.02.010
Taylor, G. C., Weyl, O. L. F., Hill, J. M., Peel, R. A., and Hay, C. J. (2017). Comparing the Fish Assemblages and Food-Web Structures of Large Floodplain Rivers. Freshw. Biol. 62, 1891–1907. doi:10.1111/fwb.13032
Veselý, L., Ercoli, F., Ruokonen, T. J., Bláha, M., Kubec, J., Buric, M., et al. (2020). The Crayfish Distribution, Feeding Plasticity, Seasonal Isotopic Variation and Trophic Role across Ontogeny and Habitat in a Canyon-Shaped Reservoir. Aquat. Ecol. 54, 1169–1183. doi:10.1007/s10452-020-09801-w
Wang, Y., Xiao, X., Yu, X., Xu, J., Cai, Y., and Lei, G. (2016). Resource Availability Determines Food Chain Length in Chinese Subtropical Rivers. Aquat. Ecol. 50, 187–195. doi:10.1007/s10452-016-9567-2
Wang, S., Luo, B.-K., Qin, Y.-J., Zhao, J.-G., Wang, T.-T., Stewart, S. D., et al. (2021a). Fish Isotopic Niches Associated with Environmental Indicators and Human Disturbance along a Disturbed Large Subtropical River in China. Sci. Total Environ. 750, 141667–141677. doi:10.1016/j.scitotenv.2020.141667
Wang, Y., Tan, W., Li, B., Wen, L., Lei, G., and Blakeslee, A. (2021b). Habitat Alteration Facilitates the Dominance of Invasive Species through Disrupting Niche Partitioning in Floodplain Wetlands. Divers. Distrib. 27, 1861–1871. doi:10.1111/ddi.13376
Wilson, K. A., Magnuson, J. J., Lodge, D. M., Hill, A. M., Kratz, T. K., Perry, W. L., et al. (2004). A Long-Term Rusty Crayfish (Orconectes Rusticus) Invasion: Dispersal Patterns and Community Change in a North Temperate Lake. Can. J. Fish. Aquat. Sci. 61, 2255–2266. doi:10.1139/f04-170
Wood, K. A., Hayes, R. B., England, J., and Grey, J. (2017). Invasive Crayfish Impacts on Native Fish Diet and Growth Vary with Fish Life stage. Aquat. Sci. 79, 113–125. doi:10.1007/s00027-016-0483-2
Wu, N., Wang, Y., Wang, Y., Sun, X., Faber, C., and Fohrer, N. (2022). Environment Regimes Play an Important Role in Structuring Trait-and Taxonomy-Based Temporal Beta Diversity of Riverine Diatoms. J. Ecol.. doi:10.1111/1365-2745.13859
Wu, Z., Zhang, D., Cai, Y., Wang, X., Zhang, L., and Chen, Y. (2017). Water Quality Assessment Based on the Water Quality Index Method in Lake Poyang: The Largest Freshwater Lake in China. Sci. Rep. 7, 17999–18008. doi:10.1038/s41598-017-18285-y
Xu, J., Lyu, H., Xu, X., Li, Y., Li, Z., Lei, S., et al. (2019). Dual Stable Isotope Tracing the Source and Composition of POM during Algae Blooms in a Large and Shallow Eutrophic Lake: All Contributions from Algae? Ecol. Indic. 102, 599–607. doi:10.1016/j.ecolind.2019.03.014
Zhang, H., Wu, G., Zhang, H., Xie, P., Xu, J., and Zhou, Q. (2013). Role of Body Size and Temporal Hydrology in the Dietary Shifts of Shortjaw Tapertail Anchovy Coilia Brachygnathus (Actinopterygii, Engraulidae) in a Large Floodplain Lake. Hydrobiologia 703, 247–256. doi:10.1007/s10750-012-1370-z
Zhang, Y. P., Chen, W. J., Fang, C. L., Zhou, H. M., He, G., Wu, B., et al. (2014). Analysis of Population Structure of Procambarus Clarkii in Poyang Lake. Jiangxi Fish. Sci. Technol. 2, 6–9+19. (in Chinese with English abstract).
Zhang, H., Kang, M., Shen, L., Wu, J., Li, J., Du, H., et al. (2020). Rapid Change in Yangtze Fisheries and its Implications for Global Freshwater Ecosystem Management. Fish. Fish. 21, 601–620. doi:10.1111/faf.12449
Keywords: basal resource, MixSIAR model, stable isotope, trophic niche, Yangtze River
Citation: Wu J, Chen H, Jin B, Winemiller KO, Wu S, Xu W, Zhang H and Wu X (2022) Seasonal Variation in Resource Overlap Between Red Swamp Crayfish (Procambarus clarkii) and Native Species in Poyang Lake Wetland, China. Front. Environ. Sci. 10:923962. doi: 10.3389/fenvs.2022.923962
Received: 20 April 2022; Accepted: 12 May 2022;
Published: 31 May 2022.
Edited by:
Shaoda Liu, Beijing Normal University, ChinaReviewed by:
Naicheng Wu, Ningbo University, ChinaPeter Mervyn Negus, Queensland Government, Australia
Copyright © 2022 Wu, Chen, Jin, Winemiller, Wu, Xu, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huili Chen, huilichen@hznu.edu.cn; Binsong Jin, jin.binsong@gmail.com
 Jiajia Wu
Jiajia Wu Huili Chen1*
Huili Chen1*  Binsong Jin
Binsong Jin Kirk O. Winemiller
Kirk O. Winemiller Xiaoping Wu
Xiaoping Wu