Abstract
Baricitinib (Olumiant®) is an oral small molecule inhibitor of Janus kinase (JAK)1 and JAK2, which have been implicated in the pathogenesis of atopic dermatitis (AD). In phase III studies in adults with moderate to severe AD who were inadequately controlled with topical corticosteroids (TCS) or systemic treatments (e.g. ciclosporin), or for whom these therapies were not advisable, baricitinib, alone or in combination with TCS, achieved significant and/or clinically relevant improvements in multiple measures of disease severity, pruritus, skin pain, sleep disturbance and health-related quality of life (HR-QOL) over 16 weeks. Benefit onset was rapid, with efficacy generally sustained over the longer term (treatment duration ≤ 68 weeks). In this patient population, the safety profile of baricitinib was consistent with that established in the moderate to severe rheumatoid arthritis (RA) population. Although further longer-term data would be beneficial, current evidence indicates that baricitinib, alone or in combination with TCS, provides an oral alternative to subcutaneous biologics for the treatment of moderate to severe AD in adults who are candidates for systemic therapy.
Plain Language Summary
A better understanding of the multiple factors that cause atopic dermatitis (AD; a chronic, relapsing, inflammatory skin disease often known as eczema) has led to the development of novel therapies that target various inflammatory pathways involved in the disease process. Baricitinib (Olumiant®), a Janus kinase (JAK)1 and JAK2 inhibitor that targets inflammatory pathways in AD, is a once-daily oral treatment approved in the EU for moderate to severe AD in adults who are candidates for systemic therapy. In such patients, baricitinib, alone or in combination with topical corticosteroids, improved disease severity, pruritus, skin pain, sleep disturbance and health-related quality of life compared with placebo over 16 weeks. Benefit onset was rapid and generally sustained over the longer term (treatment duration ≤ 68 weeks). The safety profile of baricitinib in patients with moderate to severe AD is consistent with that seen in adults with moderate to severe rheumatoid arthritis treated with the drug. Thus, baricitinib provides a convenient oral alternative to subcutaneous biologics for the treatment of moderate to severe AD.
Similar content being viewed by others
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.19184912. |
Convenient once daily oral formulation |
Can be used with or without TCS (or topical calcineurin inhibitors for sensitive areas) |
Provides early and sustained improvements in multiple measures of disease severity, pruritus, skin pain, sleep disturbance and HR-QOL |
Safety profile consistent with that established in patients with moderate to severe RA |
1 Introduction
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disorder characterized by eczematous skin lesions and pruritus [1, 2]. While predominately a paediatric disease, it may persist into or develop in adulthood [2]. Up to one-fifth of patients with AD develop moderate to severe disease, which is often refractory to first-line therapies [such as emollients, topical corticosteroids (TCS) and topical calcineurin inhibitors (TCIs)] and can result in poor quality of life [3, 4]. In such patients, phototherapy and systemic agents (e.g. oral corticosteroids) are recommended; however, the long-term use of both is not advocated owing to well-known adverse events (AEs) [1, 2, 5].
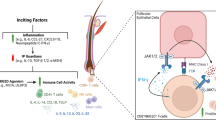
Progress in understanding the multifactorial pathogenesis of and the multiple immune pathways involved in AD has resulted in the development of novel biologics and small molecule therapies that target various inflammatory mediators implicated in the disease process, including the T helper lymphocyte (Th) type 2 cytokines IL-4, IL-13 and IL-31 (IL-31 being a key participant in the induction of pruritus), and the Th type 22 cytokine IL-22 [3, 6]. Many of these cytokines rely on the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signalling pathway, with JAK inhibition reducing the phosphorylation and activation of STATs, which activate cellular gene expression [3, 7].
Oral baricitinib (Olumiant®) was the first JAK inhibitor to be approved in the EU for the treatment of moderate to severe AD in adults who are candidates for systemic therapy [7]. This article discusses pharmacological, therapeutic efficacy and tolerability data relevant to its use in this patient population. The use of baricitinib in adults with rheumatoid arthritis (RA) has been summarized previously [8] and is beyond the scope of this review.
2 Pharmacodynamic Properties of Baricitinib
The JAK family comprises JAK1, JAK2, JAK3 and tyrosine kinase 2 (Tyk2), with each member selectively binding to different receptors [9]. Targeting JAK1 and JAK2 inhibits (to varying degrees) all cytokine receptors containing the γc chain, βc common and gp130, along with interferons, IL-12, IL-23, IL-27 and the hormone-like cytokines [9].
Baricitinib is a potent, selective and reversible small molecule inhibitor of JAK1 and JAK2 [7]. In vitro, it potently inhibited these kinases [half maximal inhibitory concentration (IC50) 5.9 and 5.7 nmol/L], displayed less activity against JAK3 and Tyk2 (IC50 ≈ 560 and 53 nmol/L) and did not inhibit the (unrelated) Chk2 and c-Met kinases (IC50 > 1000 and > 10,000 nmol/L) [7, 10]. In peripheral blood mononuclear cells (PBMCs), baricitinib potently (IC50 ≈ 20–50 nmol/L) inhibited the IL-6-stimulated phosphorylation of STAT3 and the subsequent production of the inflammatory chemokine monocyte chemoattractant protein-1, and the IL-23-induced phosphorylation of STAT3 and the subsequent production of the pathogenic cytokines IL-17 and IL-22 [10]. At concentrations of up to 10 µmol/L, baricitinib had no effect on Ba/F3-TEL-JAK3 cell proliferation, while compounds structurally similar to baricitinib but lacking its JAK1 and JAK2 inhibitory activity displayed no significant effect in PBMCs [10]. In a molecular analysis of draining lymph nodes from a rat adjuvant-induced model of arthritis, oral baricitinib (10 mg/kg once daily) reduced the expression of inflammatory Th1-associated (IL-12 and IFNγ) and Th17-associated (IL-17 and IL-22) cytokines by 55% to ≈ 80% [10].
Baricitinib inhibited (in a time and dose-dependent manner) cytokine (IL-6 or thrombopoietin)-induced STAT3 phosphorylation in the whole blood of healthy adults; maximal inhibition was seen 1–2 h post dose (concurrent with the maximum concentration), with levels generally returning to baseline values by 16–24 h [11]. In in vitro human skin models treated with IL-4, IL-13 and IL-31, it reduced the expression of phosphorylated STAT in epidermal keratinocytes, increased the expression of filaggrin (FLG; a protein that plays a role in skin barrier function and the pathogenesis of AD, with FLG loss of function mutations conferring a strong susceptibility to AD [12]) and reduced cytokine-stimulated AD-like histopathology (e.g. diminished granular cell layer and increased spongiosis accompanied by a reduction in filaggrin immunostaining) [7, 13].
Like other JAK inhibitors, baricitinib is associated with a transient reduction in absolute neutrophil count (ANC) [11]. In healthy adults, ANCs were dose-dependently reduced: nadir was reached 8 h post dose and a recovery to baseline 12–24 h post dose. As this rapid return to baseline was seen following both single and multiple doses, the reduction in ANC is unlikely to be the result of bone marrow suppression. As with other JAK inhibitors, baricitinib induces a transient increase in absolute lymphocyte count; in healthy adults, values peaked at ≈ 6 h post dose and returned to normal by 24 h post dose [11].
In patients with AD, baricitinib reduced cystatin C levels (which can be used to estimate GFR) by 0.1 mg/L at week 4; there was no further reduction seen up to week 16 [7].
3 Pharmacokinetic Properties of Baricitinib
Food has no clinically relevant effect on the pharmacokinetics of baricitinib [11] (Sect. 6). Following oral administration, the drug is rapidly absorbed (its concentration peaking a median of ≈ 1 h post dose) and has an absolute bioavailability of 79% [7, 11]. Following repeated once daily dosing in healthy adults, systemic exposure was linear and independent of time over a 2–20 mg dose range, there was minimal systemic accumulation and steady-state was reached within 48 h of the first dose [11]. Baricitinib is ≈ 50% bound to plasma proteins [7].
Metabolism (by CYP3A4) accounts for < 10% of an administered baricitinib dose [7]. Baricitinib is mostly eliminated via glomerular filtration and active secretion via BCRP, OAT3, P-gp, and multidrug and toxic extrusion protein (MATE)2-K (≈ 75% of the administered dose) and in the faeces (≈ 20%), mainly as the unchanged drug (84% of the administered dose; ≈ 6% is excreted as oxidative metabolites). In patients with AD, the mean half-life of baricitinib was 12.9 h [7].
Given its mainly renal route of elimination, baricitinib exposure is substantially affected by renal function [7]. A 2 mg (reduced from 4 mg; Sect. 6) once daily dosage of baricitinib is considered appropriate for patients with a CLCR of 30–60 mL/min, with baricitinib use not recommended in patients with a CLCR of < 30 mL/min. Baricitinib has not been studied in patients with severe hepatic impairment and its use in these patients is not recommended [7]. The pharmacokinetics of baricitinib are comparable between healthy Chinese and healthy Caucasian adults [14].
In vitro, baricitinib is a substrate for CYP3A4, BCRP, OAT3, P-gp and MATE2-K [7]. Coadministration of baricitinib and probenecid (an OAT3 inhibitor with strong inhibition potential) resulted in an ≈ 2-fold increase in baricitinib exposure. Thus, a 2 mg once daily dosage is recommended for patients taking strong OAT3 inhibitors (Sect. 6). Caution is recommended when leflunomide, or its active form teriflunomide (a weak OAT3 inhibitor) are coadministered with baricitinib, as there may be an increase in baricitinib exposure [7].
4 Therapeutic Efficacy of Baricitinib
The potential of oral baricitinib in adults with moderate to severe AD inadequately controlled by TCS was demonstrated in a multinational, phase II study [15]. This section focuses on the subsequent phase III clinical programme, which is evaluating the therapeutic efficacy of baricitinib as monotherapy or in combination with TCS in adults with moderate to severe AD participating in randomized, double-blind, multinational studies of 16 weeks’ duration (BREEZE-AD1 [16], BREEZE-AD2 [16] and BREEZE-AD7 [17]) and 104 weeks’ duration (BREEZE-AD3 [18], BREEZE-AD4 [19] and BREEZE-AD5 [20]), and a noncomparative, multinational study of 104 weeks’ duration (BREEZE-AD6 [21]).
BREEZE-AD1, BREEZE-AD2, BREEZE-AD5 and BREEZE-AD7 enrolled adults with moderate to severe AD [defined as a validated Investigator’s Global Assessment of AD (vIGA-AD) score of ≥ 3, an Eczema Area and Severity Index (EASI) score of ≥ 16 and a body surface area involvement of ≥ 10%] who had experienced an inadequate response to TCS [16, 17, 20] or systemic immunosuppressant therapies [16, 17], or had an intolerance to TCS [16, 20]. Patients who had experienced a venous thromboembolic event (VTE) or major adverse cardiovascular event (MACE) within 12 weeks of screening [16, 17, 20], had a history of VTE or recurrent VTEs [16, 17, 20] or had experienced an important AE to TCS [17] were among those excluded. BREEZE-AD4 enrolled adults with moderate to severe AD who had failed, or had an intolerance or contraindication to ciclosporin [19].
BREEZE-AD3 enrolled patients who had completed the final active treatment visit in BREEZE-AD1, BREEZE-AD2 or BREEZE-AD7 [18]; only results for patients originating from BREEZE-AD1 and BREEZE-AD2 are available. BREEZE-AD6 enrolled patients who were partial responders or non-responders at week 16 of BREEZE-AD5, with any BREEZE-AD5 responders who later lost their response or completed week 104 also eligible for BREEZE-AD6 [21].
Following a 2-week (for topical treatments) or 4-week (for systemic treatments) washout period [16,17,18,19,20, 22], patients received once-daily baricitinib (1, 2 or 4 mg) [16,17,18,19,20,21] or placebo [16,17,18,19,20], as monotherapy [16, 18, 20] or in combination with low and/or moderate potency TCS [21] (or TCIs or crisaborole where approved [17]) for active lesions [17, 19]. The 1 mg dose of baricitinib was found to be no more effective than placebo [16, 20]; thus, discussion focuses on the approved EU doses of 2 and 4 mg. Emollient use was required throughout the studies [16,17,18,19,20,21,22], and patients could receive topical and/or systemic (rescue) therapy (at the investigator’s discretion [17] at any time [16, 18,19,20, 22]) if they experienced worsening or unacceptable AD symptoms. While patient demographics and clinical characteristics were similar among the treatment groups at baseline [16, 17, 20], 41.9% of 497 patients in BREEZE-AD1 and 50.2% of 490 patients in BREEZE-AD2 had a baseline vIGA-AD score of 4 (i.e. severe disease) [16].
4.1 Short-Term Efficacy
4.1.1 Monotherapy
Monotherapy with baricitinib for 16 weeks improved multiple measures of disease severity, pruritus, skin pain, sleep disturbance and health-related quality of life (HR-QOL) in adults with moderate to severe AD participating in BREEZE-AD1, BREEZE-AD2 and BREEZE-AD5 [16, 20].
At week 16, baricitinib 4 mg demonstrated a statistically significant and clinically relevant advantage over placebo for the primary and key secondary disease severity endpoints in BREEZE-AD1 and BREEZE-AD2, reflecting a ≥ 3.7-fold higher likelihood of achieving a vIGA-AD (primary endpoint), EASI75, EASI90 or SCORing Atopic Dermatitis index (SCORAD)75 response with baricitinib 4 mg than placebo (see Table 1 for definitions and results). Significant (nominal p ≤ 0.05 vs placebo) differences were seen at each timepoint from week 1 for the EASI75 response and the change in EASI score in both studies, from week 2 for the vIGA-AD response in BREEZE-AD2, and from week 4 for the vIGA-AD response in BREEZE-AD1 and the EASI90 and SCORAD75 responses in both studies [16]. Results for baricitinib 2 mg were generally similar to those for baricitinib 4 mg: the primary endpoint was met in BREEZE-AD1, BREEZE-AD2 and BREEZE-AD5, and all key secondary disease severity endpoints were significantly improved versus placebo at week 16 in BREEZE-AD2 and BREEZE-AD5 (Table 1). However, among key secondary disease severity endpoints in BREEZE-AD1, only EASI75 was shown to be significant when adjusting for multiplicity (Table 1). Significant (nominal p ≤ 0.05 vs placebo) differences were seen at each timepoint from week 1 for the change in EASI score in BREEZE-AD1 and BREEZE-AD2, from week 2 for the EASI75 response in all three studies, from week 4 for the EASI90 response in BREEZE-AD2 and the vIGA-AD response in BREEZE-AD1, and from week 12 for the SCORAD75 response in BREEZE-AD2 [16, 20].
In terms of patient-reported key secondary endpoints, significantly more baricitinib 4 mg than placebo recipients achieved a clinically relevant (i.e. ≥ 4-point improvement from baseline [23]) Itch Numerical Rating Scale (NRS) response at week 16 in BREEZE-AD1 and BREEZE-AD2 (Table 2). Baricitinib 4 mg was also associated with significant improvements from baseline at week 16 in skin pain (assessed by Skin Pain NRS) and sleep disturbance (assessed by Item 2 of the AD Sleep Scale) in both studies (Table 2). For all of these endpoints, significant (p ≤ 0.05 vs placebo; values unadjusted for multiplicity unless otherwise specified) differences were seen at each weekly timepoint from week 1 (adjusted p ≤ 0.05 for the Itch NRS response and the change in Item 2 of the ADSS at week 1 and adjusted p ≤ 0.001 for the Itch NRS response at weeks 2 and 4) [16]. Baricitinib 2 mg was significantly more effective than placebo as regards the proportion of patients achieving a clinically relevant Itch NRS response at week 16, and was associated with significant improvements from baseline at week 16 in skin pain and sleep disturbance in BREEZE-AD2 and BREEZE-AD5, but not in BREEZE-AD1 (Table 2). Significant (p ≤ 0.05 vs placebo; values unadjusted for multiplicity unless otherwise specified) differences were seen at each weekly timepoint from week 2 for the Itch NRS response in BREEZE-AD2 and BREEZE-AD5 and from week 1 for the changes in skin pain and sleep disturbance in BREEZE-AD2 (adjusted p ≤ 0.01 for the Itch NRS response at weeks 2 and 4 and adjusted p ≤ 0.05 for the change in sleep disturbance at week 1) [16, 20].
With regard to other patient-reported outcomes, nominally significant differences favouring baricitinib 4 mg over placebo at week 16 in BREEZE-AD1 and BREEZE-AD2 were seen in the proportions of patients achieving clinically relevant (i.e. ≥ 4-point and ≥ 3.4-point improvement from baseline [23]) skin pain and Patient-Oriented Eczema Measure (POEM) responses and improvements in itch (as assessed by the Itch NRS) and the POEM total score (Table 2). At week 16 in both studies, baricitinib 4 mg also significantly (nominal p ≤ 0.001 vs placebo) improved the proportion of patients achieving Dermatology Life Quality Index (DLQI) total scores of 0 or 1, or ≤ 5, and was associated with a significant (nominal p ≤ 0.001 vs placebo) and clinically relevant (minimal clinically important difference of 4 points [23]) mean improvement from baseline in the DLQI total score [16, 24]. DLQI total scores range from 0–30; scores of 0 or 1 indicate no effect and a score of ≤ 5 represents no to a small effect on HR-QOL [16, 24]. In a post hoc analysis of data from the first week of therapy in BREEZE-AD1 and BREEZE-AD2, baricitinib 4 mg significantly (nominal p ≤ 0.01 vs placebo) improved itch and sleep disturbance as early as day 2 (i.e. one day after the first dose), with these improvements maintained to day 7 [25]. Baricitinib 2 mg was associated with nominally significant between-groups differences relative to placebo in the proportions of patients achieving a clinically relevant POEM response (Table 2) and a DLQI total score of 0 or 1 response (nominal p ≤ 0.05) at week 16 in BREEZE-AD1 and BREEZE-AD2 [16, 24]. In BREEZE-AD1, BREEZE-AD2 and BREEZE-AD5, it also provided nominally significant improvements in itch (Table 2), the POEM total score (Table 2) and the DLQI total score (nominal p ≤ 0.05) compared with placebo at week 16 [16, 20, 24]. A clinically relevant Skin Pain NRS response (Table 2) and a DLQI total score of ≤ 5 response (nominal p ≤ 0.001) was achieved in significantly more baricitinib 2 mg than placebo recipients at week 16 in BREEZE-AD2, but not BREEZE-AD1 [16, 24]. Baricitinib 2 mg significantly (nominal p ≤ 0.05 vs placebo) improved itch from baseline as early as day 2 in BREEZE-AD1 and BREEZE-AD2 and sleep disturbance from baseline as early as day 4 in BREEZE-AD2, with these improvements maintained to day 7, according to a post hoc analysis of data from the first week of therapy in these studies [25].
4.1.2 Combination Therapy
Combination therapy with baricitinib plus TCS for 16 weeks improved the signs and symptoms of AD to a greater extent than TCS in adults with moderate to severe disease participating in BREEZE-AD7 [17].
At week 16, baricitinib 4 mg plus TCS was significantly more effective than placebo plus TCS as regards the proportions of patients achieving vIGA-AD (primary endpoint) and EASI75 responses, and was associated with a significant reduction from baseline in the EASI score (Table 1). However, the SCORAD75 and EASI90 responses were nominally significant (Table 1). The primary endpoint was not met for baricitinib 2 mg plus TCS; nominal significance was shown for two key secondary disease severity endpoints (Table 1).
In terms of patient-reported key secondary endpoints, baricitinib 4 mg plus TCS was associated with a significant and clinically relevant Itch NRS response at week 16 compared with placebo plus TCS (Table 2). Significant between-group differences favouring baricitinib 4 mg plus TCS in this endpoint were seen at week 2 (33% vs 15%; nominal p = 0.001) and week 4 (52% vs 11%; p < 0.001), but not at earlier timepoints [17]. Baricitinib 4 mg plus TCS was also associated with significant improvements in skin pain at week 16 (Table 2), and in sleep disturbance at week 1 (− 0.9 vs − 0.5; nominal p = 0.002) and week 16 (Table 2). Compared with placebo plus TCS, baricitinib 2 mg plus TCS was associated with significant between-group differences in the proportion of patients achieving a clinically relevant Itch NRS response at week 4 (34.0% vs 10.6%; nominal p < 0.001) and week 16 (Table 2), but not at earlier timepoints, and in improvements in skin pain and sleep disturbance at week 16 (Table 2), but not improvements in sleep disturbance at week 1 [17].
With regard to other patient-reported outcomes, significantly (nominal p ≤ 0.01) more baricitinib 4 mg plus TCS than placebo plus TCS recipients achieved a clinically relevant ≥ 4-point improvement from baseline in the DLQI total score, a DLQI total score of 0 or 1 and a DLQI total score of ≤ 5 at week 16 [26]. Significant (nominal p ≤ 0.01 vs placebo plus TCS) between-group differences in these endpoints were seen at week 2 and all subsequent timepoints thereafter [26]. Baricitinib 4 mg plus TCS also provided a nominally significant improvement from baseline in itch (Table 2), and nominally significant changes from baseline in the POEM total score (Table 2) and the DLQI total score (nominal p < 0.001) compared with placebo plus TCS at week 16 [17]. In a post hoc analysis of data from the first week of therapy in BREEZE-AD7, baricitinib 4 mg plus TCS was associated with statistically significant (nominal p ≤ 0.05 vs placebo) improvements in itch and sleep disturbance as early as day 2, with these improvements maintained through to day 7 [25]. Significantly (nominal p ≤ 0.05) more baricitinib 2 mg plus TCS than placebo plus TCS recipients achieved DLQI total score of 0 or 1 and DLQI total score of ≤ 5 responses, but not a clinically relevant ≥ 4-point improvement from baseline in the DLQI total score response, at week 16 [26]. Significant (nominal p ≤ 0.05 vs placebo plus TCS) differences were seen from week 2 for the DLQI total score of ≤ 5 response and from week 4 for the DLQI total score of 0 or 1 response [26]. Baricitinib 2 mg plus TCS was also associated with a nominally significant improvement in itch (Table 2) and nominally significant changes from baseline in the POEM total score (Table 2) and the DLQI total score (nominal p < 0.001 vs placebo plus TCS) at week 16 [17]. Baricitinib 2 mg plus TCS significantly (nominal p ≤ 0.05 vs placebo plus TCS) improved itch as early as day 4 and sleep disturbance at day 6, with these improvements maintained to day 7, according to a post hoc analysis of data from the first week of therapy in BREEZE-AD7 [25].
During BREEZE-AD7, 6 of 111 baricitinib 4 mg plus TCS recipients, 5 of 109 baricitinib 2 mg plus TCS recipients and 10 of 109 placebo plus TCS recipients required rescue therapy with high or ultra-high potency TCS, or systemic therapies [17].
Results from BREEZE-AD7 are supported by preliminary data from BREEZE-AD4 in adults with moderate to severe AD who have failed, or who have an intolerance or contraindication to ciclosporin [19]. At week 16, significantly more baricitinib 4 mg plus TCS (n = 92) than placebo plus TCS (n = 93) recipients achieved an EASI75 response (32% vs 17%; p = 0.031) [primary endpoint], with the mean percentage change from baseline in the EASI score significantly greater with baricitinib 4 mg than placebo (− 63.3 vs − 42.7; p < 0.001) [19]. Of the remaining disease severity endpoints (EASI90, vIGA-AD and SCORAD75 responses), only the vIGA-AD response demonstrated a significant between-group difference (22% vs 10%; nominal p = 0.03). The primary endpoint was not met for baricitinib 2 mg plus TCS (n = 185) versus placebo plus TCS (28% vs 17%); significance was demonstrated for two of the four key secondary disease severity endpoints [mean percentage change from baseline in the EASI score: − 56.1 vs − 42.7, nominal p = 0.006; SCORAD75 response: 8% vs 1%, nominal p = 0.037] [19].
In terms of patient-reported key secondary endpoints, a clinically relevant Itch NRS response (assessed in patients with a baseline Itch NRS score of ≥ 4: n = 76 and 85, respectively) was significantly (p ≤ 0.002) higher with baricitinib 4 mg plus TCS than placebo plus TCS at week 2 (22% vs 5%), week 4 (41% vs 8%) and week 16 (38% vs 8%), but not week 1 (8% vs 1%) [19]. Significant (p < 0.001 vs placebo) between-group differences were also seen in the mean change from baseline in the Skin Pain NRS (− 3.0 vs − 1.6) at week 16 and in Item 2 of the ADSS at week 16 (− 1.4 vs − 0.6), but not week 1 (− 0.8 vs − 0.5). Baricitinib 2 mg plus TCS was associated with significant (nominal p < 0.05 vs placebo plus TCS) differences in the proportion of patients with a baseline Itch NRS score of ≥ 4 (n = 166 and 85, respectively) who achieved an Itch NRS response at week 2 (14% vs 5%), week 4 (24% vs 8%) and week 16 (23% vs 8%), but not week 1 (4% vs 1%), and improvements in the Skin Pain NRS (− 2.4 vs − 1.6) at week 16 [19].
With regard to other patient-reported outcomes, significant (nominal p ≤ 0.01 vs placebo plus TCS) differences were seen at week 16 in the proportions of patients achieving a DLQI total score of 0 or 1 response and a ≥ 4-point improvement from baseline in the POEM total score response and the mean changes from baseline in the POEM total score and the DLQI total score in the baricitinib 4 mg plus TCS group, and in the proportion of patients achieving a ≥ 4-point improvement from baseline in the POEM total score response and the mean change from baseline in the POEM total score in the baricitinib 2 mg plus TCS group [19].
4.2 Longer-Term Efficacy
Patients enrolled in BREEZE-AD3 were classified according to their treatment response in the originating study (BREEZE-AD1 and BREEZE-AD2): responders (those who achieved a validated vIGA-AD score of 0 or 1 and had not received rescue therapy); partial responders (those who achieved a vIGA-AD score of 2 and had not received rescue therapy); and non-responders (those who achieved a vIGA-AD score of 3 or 4 or had received rescue therapy) [18]. Responders and partial responders remained on their original treatment (and dose) during BREEZE-AD3, although those receiving baricitinib 1 mg or placebo could be re-randomized to receive baricitinib 2 or 4 mg (as rescue therapy) if they experienced disease worsening (defined as a vIGA-AD score of 3 or 4). Non-responders who had received baricitinib 1 or 2 mg, or placebo in the originating study were re-randomized to receive baricitinib 2 or 4 mg, while those who had received baricitinib 4 mg in the originating study continued to receive baricitinib 4 mg. Data from re-randomized patients were not reported. Thus, of the 1081 patients enrolled in BREEZE-AD3, 221 patients were classified as responders or partial responders (thus remaining on their original treatment and dose), with 124 receiving baricitinib 2 or 4 mg, and 860 patients were classified as non-responders, with 156 having received baricitinib 4 mg in the originating study and continuing to receive this treatment and dose. Low and moderate potency TCS use could be (re-)initiated at any time during BREEZE-AD3 and could be provided as part of rescue or re-treatment any time a patient achieved a vIGA-AD score of ≥ 3 [18].
The improvements in measures of disease severity, pruritus, skin pain and sleep disturbance seen at week 16 of BREEZE-AD1 and BREEZE-AD2 were generally sustained over the longer term (≤ 68 weeks of treatment) in BREEZE-AD3 [18].
In the responder or partial responder population receiving baricitinib 4 mg (n = 70) in BREEZE-AD3, the proportion of patients achieving a vIGA-AD score of 0 or 1 (primary endpoint) was 45.7% at week 0 (at which time patients had received 16 weeks of continuous therapy) and 47.1% at week 52 (at which time patients had received 68 weeks of continuous therapy), and the proportion of patients achieving an EASI75 response was 70.0% at week 0 and 55.7% at week 52 [18]. The mean change from baseline of the originating study in the EASI score was − 22.9 points at week 0 and − 20.0 points at week 52 of BREEZE-AD3. In the responder or partial responder population receiving baricitinib 2 mg (n = 54), 46.3% and 59.3% of patients had achieved a vIGA-AD score of 0 or 1 (primary endpoint) and 74.1% and 81.5% of patients had achieved an EASI75 response at weeks 0 and 52, respectively. The mean change from baseline of the originating study in the EASI score was − 20.4 points at week 0 and − 21.7 points at week 52 of BREEZE-AD3 [18].
With regard to patient-reported outcomes in the responder or partial responder population receiving baricitinib 4 mg at weeks 0 and 16, respectively, of BREEZE-AD3, 52.5% and 45.9% of patients had achieved a clinically relevant Itch NRS response, 61.8% and 54.5% had achieved a clinically relevant Skin Pain NRS response and 75.0% and 71.4% had achieved a ≥ 1.5-point improvement in sleep disturbance [18]. The benefits of baricitinib 4 mg on the POEM and DLQI total scores in the originating study were generally sustained at week 52 of BREEZE-AD3. At weeks 0 and 16, respectively, in the responder or partial responder population receiving baricitinib 2 mg, 44.2% and 39.5% of patients had achieved a clinically relevant Itch NRS response, 47.5% and 45.0% had achieved a clinically relevant Skin Pain NRS response and 73.7% and 73.7% had achieved a ≥ 1.5-point improvement in sleep disturbance. The benefits of baricitinib 2 mg on the POEM and DLQI total scores in the originating study were generally sustained at week 52 of BREEZE-AD3 [18].
In the responder or partial responder population of BREEZE-AD3, low and moderate potency TCS use was reported in 53% of baricitinib 4 mg and 41% of baricitinib 2 mg recipients [18].
Preliminary data from BREEZE-AD4 (n = 463) [27] and an integrated analysis (n = 146) of BREEZE-AD5 and BREEZE-AD6 [21] support the findings of BREEZE-AD3, with baricitinib 4 mg plus TCS [27] and baricitinib 2 mg plus TCS [21] associated with sustained disease control over the longer term (≤ 52 weeks of continuous treatment) in adults with moderate to severe AD. At week 52 of BREEZE-AD4, the proportions of patients achieving an EASI75 response were 37.0%, 30.3% and 26.9% in the baricitinib 4 mg, baricitinib 2 mg and placebo groups respectively; 33.8%, 22.9% and 18.8% of patients achieved an Itch NRS response (p < 0.05 for baricitinib 4 mg vs placebo) [27]. According to the integrated analysis, after 16, 32 and 52 weeks of continuous therapy, respectively, the mean percentage change from baseline in the EASI score was − 50.1%, − 59.1% and − 56.8% (mean baseline EASI score of 26.6), the proportion of patients achieving an EASI75 response was 39.6%, 51.4% and 48.6%, and the proportion of patients achieving a vIGA-AD score of 0 or 1 was 27.1%, 38.2% and 31.3%. In the mean SCORAD itch and sleeplessness scores, respectively, improvements from baseline (7.7 and 6.5 points) were seen after 16 weeks’ continuous therapy (4.8 and 3.9 points) and were maintained at week 32 (3.8 and 3.4 points) and week 52 (4.3 and 3.7 points). A small or no effect on HR-QOL by AD was seen with baricitinib 2 mg plus TCS, with 38.6%, 48.8% and 44.9% of 129 patients with a baseline DLQI total score of > 5 having DLQI total scores of ≤ 5 at weeks 16, 32, and 52, respectively [21].
5 Safety and Tolerability of Baricitinib
The safety profile of baricitinib in adults with moderate to severe AD is consistent with that seen in the moderate to severe RA population, according to an integrated analysis [28] of eight studies (the phase II study [15] and the seven phase III BREEZE-AD studies [16,17,18,19,20,21]): no new safety signals were reported [23]. No clear clinically relevant differences were seen when baricitinib was administered alone or in combination with TCS, and there were no obvious differences between the 2 and 4 mg once daily dosages [23].
The integrated analysis included data from 2531 patients (mean age of 36.4 years) treated with ≥ 1 dose of baricitinib (1, 2 or 4 mg, once daily), representing 2247 patient–years of exposure (PYE) [all baricitinib AD dataset]; 42% of patients in this dataset had ≥ 1 year of exposure to baricitinib [28]. Two further datasets analyzed the safety profile of baricitinib 2 or 4 mg: during the 16-week placebo-controlled periods of the phase II study and BREEZE-AD1, BREEZE-AD2, BREEZE-AD4 and BREEZE-AD7 (placebo-controlled dataset; ≤ 211.8 PYE); and utilizing data from the placebo-controlled dataset and BREEZE-AD3 (extended dataset; ≤ 459.3 PYE). In the extended dataset, 30% and 43% of patients who had received baricitinib 2 mg or 4 mg had ≥ 1 year of exposure. Percentage values are study-size adjusted in the placebo-controlled dataset; incidence rates (IRs) are study-size adjusted in the placebo-controlled and extended datasets [28]. Subsequent discussion focuses on the 2 and 4 mg doses.
In the placebo-controlled dataset, treatment-emergent AEs (TEAEs) [most were mild or moderate in severity] were reported in 51.0% of 489 patients receiving baricitinib 4 mg, 49.3% of 576 patients receiving baricitinib 2 mg and 43.2% of 743 patients receiving placebo [28]. The most frequently reported individual TEAEs in the baricitinib 4 mg, baricitinib 2 mg and placebo groups, respectively, were nasopharyngitis (11.3%, 9.5% and 9.5% of patients), headache [6.3%, 5.9% and 3.3%; most were mild in severity and of short (median < 1 day) duration], elevations in blood creatine phosphokinase (CPK) levels (2.9%, 1.1% and 0.8%) and diarrhoea (2.7%, 1.3% and 1.8%). Generally, the elevations in CPK levels were transient, asymptomatic and not associated with muscle-related symptoms; there were no cases of rhabdomyolysis. Therapy with baricitinib was associated with small, reversible and dose-dependent increases in serum creatinine levels; however, there was no evidence of an increased risk of serious renal AEs. While increases in high and low-density lipoprotein cholesterol levels were seen, the low occurrence of MACE and other cardiovascular risks (Sect. 5.1) do not suggest an increase in the risk of these AEs. Changes in haemoglobin levels and in lymphocyte, neutrophil and platelet counts were minimal [28].
Serious AEs (SAEs; most were AD) occurred in 2.3% of baricitinib 4 mg recipients, 1.4% of baricitinib 2 mg recipients and 2.3% of placebo recipients (placebo-controlled dataset) [28]. In the respective groups, treatment interruptions due to an AE (most commonly an infection) occurred in 4.6%, 3.4% and 1.6% of patients, with few patients discontinuing therapy due to an AE (2.1%, 1.5% and 1.4% of patients). There were no deaths in the placebo-controlled dataset [28].
In the extended versus placebo-controlled datasets, similar or lower IRs were displayed by both doses of baricitinib for TEAEs [4 mg: 248.3 vs 300.1 per 100 patients-years at risk (PYR); 2 mg: 237.3 vs 281.4 PYR], treatment interruptions due to an AE (4 mg: 12.7 vs 15.8 PYR; 2 mg: 10.4 vs 11.6 PYR) and treatment discontinuations due to an AE (4 mg: 5.5 vs 6.5 PYR; 2 mg: 3.6 vs 4.7 PYR) [28]. However, SAE IRs were higher for baricitinib 4 mg (9.1 vs 7.7 PYR) but lower for baricitinib 2 mg (3.5 vs 4.4 PYR) in the extended dataset versus the placebo-controlled dataset. No deaths were reported in the extended dataset. One patient in the all baricitinib AD dataset died due to gastrointestinal bleeding. The patient had received > 12 months’ baricitinib therapy (initially 1 mg in an originating study, then 4 mg in an extension study, which was adjusted to 2 mg owing to a reduced glomerular filtration rate) [28].
5.1 Adverse Events of Special Interest
In general, the pattern of AEs of special interest (AESIs) with baricitinib therapy was similar between the AD and RA populations [23].
Owing to a defective skin barrier, and immunological dysregulation (Sect. 1), patients with AD have an increased risk of bacterial and viral infections (both cutaneous and non-cutaneous) [28]. Moreover, via its inhibitory effect on the JAK–STAT pathway (Sect. 2), baricitinib is surmised to increase the risk of infection [23]. In the placebo-controlled dataset, treatment-emergent infections (most were mild or moderate in severity) occurred in 31.5% of baricitinib 4 mg recipients, 29.8% of baricitinib 2 mg recipients and 24.2% of placebo recipients [28]. Baricitinib 4 mg and 2 mg were both associated with a low incidence of serious infections (0.6% and 0.4% vs 0.6% with placebo) and opportunistic infections (excluding tuberculosis) [0% and 0.1% vs 0.1%]. There was also a low incidence of eczema herpeticum (based on a cluster containing the preferred terms eczema herpeticum and Kaposi’s varicelliform eruption) [0.2–1.4% across baricitinib 4 mg or 2 mg and placebo recipients] and herpes zoster (0–0.8%). Herpes simplex (based on a cluster of preferred terms) was reported in 6.1% of baricitinib 4 mg recipients, 3.6% of baricitinib 2 mg recipients and 2.7% of placebo recipients and skin infections requiring antibacterial therapy in 3.4%, 4.8% and 4.4% of patients, respectively. There were no cases of tuberculosis reported [28].
Severe, and predominately active AD is independently associated with an increased risk of cardiovascular events, and thromboembolic events are a recognized AE of JAK inhibitors [28]. In the placebo-controlled dataset, there were no reports of positively adjudicated MACE or other cardiovascular events; however, one baricitinib 4 mg recipient experienced a VTE [pulmonary embolism (PE)]. No malignancies were reported with either baricitinib dose, but two malignancies other than non-melanoma skin cancer (NMSC) [IR 0.66] and one NMSC (IR 0.68) occurred with placebo. The incidence of conjunctival disorders (1.2% for baricitinib 4 mg, 1.6% for baricitinib 2 mg and 2.1% with placebo) was low in the placebo-controlled dataset, and no gastrointestinal perforations were reported [28].
In the extended versus placebo-controlled datasets, similar or lower IRs were displayed by both doses of baricitinib for treatment-emergent infections (4 mg: 117.4 vs 134.5 PYR; 2 mg: 115.4 vs 128.0 PYR), the herpes simplex cluster (4 mg: 14.5 vs 21.3 PYR; 2 mg: 9.6 vs 12.4 PYR) and skin infections requiring antibacterial therapy [4 mg: 4.3 vs 11.4 PYR; 2 mg: 7.5 vs 16.7 PYR], but not for serious infections (4 mg: 3.0 vs 1.9 PYR; 2 mg: 1.5 vs 1.0 PYR), opportunistic infections (4 mg: 0.3 vs 0 PYR; 2 mg: 0.2 vs 0.3 PYR) or herpes zoster (4 mg: 1.8 vs 0 PYR; 2 mg: 3.8 vs 2.7 PYR) [28]. The eczema herpeticum cluster IRs were lower for baricitinib 4 mg (2.6 vs 4.5 PYR) but higher for baricitinib 2 mg (1.1 vs 0.7 PYR] in the extended versus placebo-controlled datasets. No cases of tuberculosis were reported in the extended and all baricitinib AD datasets. One positively adjudicated MACE, a myocardial infarction in a patient with multiple cardiovascular risk factors, was reported in the baricitinib 2 mg group in the extended dataset (IR 0.17). One additional MACE, a haemorrhagic stroke (in a patient who had received placebo in an originating study and who was then switched to baricitinib 2 mg in an extension study) was reported in the all baricitinib AD dataset (IR 0.09). Two VTEs (both PE) were reported in the baricitinib 4 mg group in the extended and all baricitinib AD datasets (IRs 0.40 and 0.09). No malignancies were reported with either baricitinib dose in the extended dataset. In the all baricitinib AD dataset, there were five malignancies other than NMSC (one in the baricitinib 4 mg group and four in the baricitinib 2 mg group) [IR 0.22] and six NMSCs (three in the baricitinib 4 mg group and three in the baricitinib 2 mg group) [IR 0.26]. The conjunctival disorders IRs were higher for baricitinib 4 mg (4.6 vs 3.7 PYR) but lower for baricitinib 2 mg (4.9 vs 5.6 PYR) in the extended versus placebo-controlled datasets. No gastrointestinal perforations were reported in the extended and all baricitinib AD datasets [28].
6 Dosage and Administration of Baricitinib
Baricitinib is approved for the treatment of moderate to severe AD in adults who are candidates for systemic therapy in numerous countries worldwide, including those of the EU [7]. The recommended dosage of baricitinib is 4 mg once daily (with or without food). A 2 mg dose is considered appropriate for patients aged ≥ 75 years, those taking strong OAT3 inhibitors (e.g. probenecid) and those with a CLCR of 30–60 mL/min; it may be appropriate for those with a history of chronic or recurrent infections and should be considered for those who have achieved sustained control of disease activity with 4 mg and are eligible for dose tapering. Baricitinib can be administered alone or in combination with TCS (or TCIs for sensitive areas). Treatment discontinuation should be considered if no evidence of therapeutic benefit is seen following 8 weeks’ therapy [7].
The use of baricitinib is contraindicated during pregnancy (as the JAK/STAT pathway is involved in cell adhesion and polarity, which can affect early embryonic development), with effective contraception required by women of child-bearing potential during and for ≥ 1 week following treatment [7]. Baricitinib should not be used in patients who are breast-feeding [7]. Local prescribing information should be consulted for detailed information regarding contraindications, warnings and precautions, potential drug interactions (e.g. with immunosuppressive medicinal products), and use in other special patient populations.
7 Place of Baricitinib in the Management of Moderate to Severe Atopic Dermatitis
Historically, the treatment options for adults with moderate to severe AD who require systemic therapy were limited to ciclosporin (approved only for intermittent use in patients with severe disease [23]) and various agents that were used off-label [5]. A better understanding of the multifactorial pathogenesis of AD has driven the development of targeted therapies [3], with the monoclonal antibody dupilumab (which inhibits IL-4 and IL-13) being the first biologic approved in the EU for adults with moderate to severe AD [29]. EU approval of other agents has followed, including the small molecule JAK inhibitors baricitinib (JAK1 and JAK2 inhibitor) [7], abrocitinib (JAK1 inhibitor) [30] and upadacitinib (JAK1 and JAK1/3 inhibitor) [31], and the monoclonal antibody tralokinumab (which inhibits IL-13) [32]. Owing to the timing of approval, only dupilumab has been incorporated into consensus-based European guidelines for the treatment of AD [5]. In the UK, the National Institute for Health and Care Excellence (NICE) recommends both dupilumab [33] and baricitinib [34] as options for adults with moderate to severe AD who have not responded to treatment with ≥ 1 systemic therapy. Dupilumab and tralokinumab are both administered subcutaneously once every other week [29, 32], while baricitinib, abrocitinib and upadacitinib are administered orally once daily [7, 30, 31]. While the specific cytokine targeting of monoclonal antibodies may give rise to a better safety profile than the broader mechanisms of action of JAK inhibitors, such targeting may render them less effective in some endotypes [35]. Baricitinib also inhibits JAK2 (Sect. 2), which modulates IL-5 [3]. IL-5 inhibition by baricitinib impedes eosinophil activation and its migration to the skin, both of which worsen AD [35]. While JAK2 inhibition has the potential to impair erythrocyte, leukocyte or platelet production, with myelosuppression seen to varying degrees with other JAK inhibitors (e.g. ruxolitinib and tofacitinib) [23], clinical findings do not appear to suggest that this will be a safety issue (Sect. 5).
Head-to-head comparisons of baricitinib with other biologic AD therapies are currently lacking. An indirect comparison of baricitinib plus TCS and dupilumab plus TCS used in the UK NICE guidance suggests that baricitinib could be less effective than dupilumab, although it should be noted that clinical experts agreed that the between-study differences in the washout period and censoring rules likely favoured dupilumab [34]. Preliminary data from another analysis that indirectly compared baricitinib and dupilumab, as monotherapies or in combination with TCS, suggested no statistically significant between-group differences in various measures of disease severity and pruritus at 16 weeks [36]. Given the limitations of indirect comparisons, the findings should be interpreted with caution. Robust head-to-head comparisons would be beneficial.
In adults with moderate to severe AD, 16 weeks’ therapy with baricitinib 4 mg, alone or in combination with TCS, provided early and sustained significant and clinically relevant improvements in multiple measures of disease severity, pruritus, skin pain, sleep disturbance and HR-QOL compared with placebo (Sect. 4.1). Generally similar results were seen with baricitinib 2 mg. Disease severity, pruritus, skin pain and sleep disturbance benefits with both doses of baricitinib were generally sustained over the longer term (≤ 68 weeks of treatment) (Sect. 4.2). As benefits with the 4 mg dose are well maintained with the 2 mg dose, lowering the dose from 4 to 2 mg is an option if a desirable target level of AD is reached [23] (Sect. 6). Indeed, preliminary data from a substudy of BREEZE-AD3 suggest that clinically relevant efficacy (as assessed by a vIGA-AD score of 0 or 1) with baricitinib was generally maintained over 16 weeks following down titration [37]. Further data from the longer-term studies and the down titration substudy are awaited with interest. Real-world data would also be useful.
Baricitinib has a safety profile in adults with moderate to severe AD consistent with that seen in the moderate to severe RA population (Sect. 5). There were no clinically relevant differences seen when baricitinib was administered alone or in combination with TCS, and no obvious differences between the 2 and 4 mg doses. The AESI pattern in the AD population was generally similar to that seen in the RA population. While infections occurred more frequently in baricitinib versus placebo recipients, the incidence of serious infections and opportunistic infections were low (Sect. 5). In patients with a history of chronic or recurrent infections, selecting the 2 mg over the 4 mg dose may be more appropriate (Sect. 6). While the incidence of malignancies, MACE and other cardiovascular events, and VTEs was low (Sect. 5), most of the studies discussed in Sect. 5 excluded patients who had recently experienced a VTE or MACE, or who had a history of VTE (see Sect. 4 for exclusion criteria), and the enrolled AD patient population only had a mean age of 36.4 years. Longer-term evaluations are still ongoing, with their data awaited with interest. In addition, MACE, VTEs, rhabdomyolysis and lipid AEs are to be closely followed in the post authorisation setting [23]. It is worth noting that while baricitinib-associated IFN suppression (Sect. 2) could be considered an impediment to treating a viral infection (e.g. COVID-19), with IFNs preventing virus replication during the early stage of an infection, such an action may alleviate the hyper-inflammation (resulting from excessive cytokine production) seen with severe COVID-19, and therefore improve clinical outcomes [38].
In the UK NICE guidance, baricitinib was reported to be cost effective (e.g. a 50% improvement from baseline in the EASI score plus a ≥ 4-point improvement in the DLQI total score) compared with dupilumab, and likely to be cost effective compared with best supportive care [34]. Moreover, incremental analyses supported the cost effectiveness of baricitinib versus dupilumab when baricitinib was used before or after dupilumab [34].
Although further longer-term efficacy and tolerability data would be beneficial, current evidence indicates that baricitinib, alone or in combination with TCS, provides an effective oral alternative to subcutaneous biologics for the treatment of moderate to severe AD in adults who are candidates for systemic therapy.
Data Selection Baricitinib: 190 records identified
Duplicates removed | 47 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 53 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 52 |
Cited efficacy/tolerability articles | 11 |
Cited articles not efficacy/tolerability | 27 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were baricitinib, Olumiant, atopic dermatitis, eczema. Records were limited to those in English language. Searches last updated 14 March 2022 | |
Change history
04 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40257-022-00717-9
References
Boytsov NN, Gorritz M, Wang X, et al. The current treatment landscape in adult atopic dermatitis in the United States: results from a cross-sectional real-world study. J Dermatolog Treat. 2021. https://doi.org/10.1080/09546634.2021.1898530.
Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–82.
Munera-Campos M, Carrascosa JM. Innovation in atopic dermatitis: from pathogenesis to treatment. Actas Dermosifiliogr (Engl Ed). 2020;111(3):205–21.
Newsom M, Bashyam AM, Balogh EA, et al. New and emerging systemic treatments for atopic dermatitis. Drugs. 2020;80(11):1041–52.
Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–78.
Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46(5):820–4.
Eli Lilly Nederland B.V. Olumiant (baricitinib) film-coated tablets: EU summary of product characteristics. 2021. https://www.ema.europa.eu/. Accessed 14 Dec 2021.
Al-Salama ZT, Scott LJ. Baricitinib: a review in rheumatoid arthritis. Drugs. 2018;78(7):761–72.
O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–28.
Fridman JS, Scherle PA, Collins R, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 2010;184(9):5298–307.
Shi JG, Chen X, Lee F, et al. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J Clin Pharmacol. 2014;54(12):1354–61.
Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease. Ann Allergy Asthma Immunol. 2020;124(1):36–43.
Nickoloff BJ, Colvin SC, Sims JT, et al. Inhibitory effects of baricitinib on a human skin model of atopic dermatitis [abstract no. P103]. Exp Dermatol. 2021;30(3): e52.
Zhao X, Sheng XY, Payne CD, et al. Pharmacokinetics, safety, and tolerability of single- and multiple-dose once-daily baricitinib in healthy Chinese subjects: a randomized placebo-controlled study. Clin Pharmacol Drug Dev. 2020;9(8):952–60.
Guttman-Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80(4):913-21.e9.
Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–55.
Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333–43.
Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157(6):691–9.
Bieber T, Reich K, Paul C, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in patients with moderate-to-severe atopic dermatitis who failed, are intolerant to, or have contraindication to cyclosporine: results from a randomized, placebo-controlled, phase 3 clinical trial (BREEZE-AD4) [abstract no. P0181]. In: 29th Congress of EADV. 2020.
Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85(1):62–70.
Simpson E, Boguniewicz M, Finklea L, et al. Long-term efficacy of baricitinib 2-mg for the treatment of atopic dermatitis in North America [abstract no. 466 plus poster]. In: Revolutionizing Atopic Dermatitis Conference (RAD). 2021.
US National Institutes of Health. ClinicalTrials.gov identifier NCT03428100. 2021. https://clinicaltrials.gov/. Accessed 10 Feb 2022.
European Medicines Agency. Olumiant (baricitinib): EU variation assessment report. 2020. https://www.ema.europa.eu/. Accessed 21 Feb 2022.
Reich K, DeLozier AM, Nunes FP, et al. Baricitinib improves symptoms in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: patient-reported outcomes from two randomized monotherapy phase III trials. J Dermatolog Treat. 2020. https://doi.org/10.1080/09546634.2020.1839008.
Buhl T, Rosmarin D, Serra-Baldrich E, et al. Itch and sleep improvements with baricitinib in patients with atopic dermatitis: a post hoc analysis of 3 phase 3 studies. Dermatol Ther (Heidelb). 2021;11(3):971–82.
Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35(7):1543–52.
Bieber T, Reich K, Paul C, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in moderate-to-severe atopic dermatitis patients with cyclosporine failure, intolerance, or contraindication: 52-week results from the BREEZE-AD 4 trial [abstract no. PT32]. Adv Dermatol Venereol. 2021;101(Suppl 221):63.
Bieber T, Thyssen JP, Reich K, et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J Eur Acad Dermatol Venereol. 2021;35(2):476–85.
sanofi-aventis groupe. Dupixent (dupilumab) solution for injection: EU summary of product characteristics 2021. https://www.ema.europa.eu/. Accessed 13 Dec 2021.
Pfizer Europe MA EEIG. Cibinqo (abrocitinib) film-coated tablets: EU summary of product characteristics. 2021. https://www.ema.europa.eu/. Accessed 11 Feb 2022.
AbbVie Deutschland GmbH & Co. KG. RINVOQ (upadacitinib) prolonged-release tablets: EU summary of product characteristics. 2021. https://www.ema.europa.eu/. Accessed 13 Dec 2021.
LEO Pharma A/S. Adtralza (tralokinumab) 150 mg solution for injection in pre-filled syringe: EU summary of product characteristics. 2021. https://www.ema.europa.eu/. Accessed 13 Dec 2021.
UK National Institute for Health Excellence. Dupilumab for treating moderate to severe atopic dermatitis: technology appraisal guidance (TA534). 2018. https://www.nice.org.uk/guidance/ta534. Accessed 15 Dec 2021.
UK National Institute for Health Excellence. Baricitinib for treating moderate to severe atopic dermatitis: technology appraisal guidance (TA681). 2021. https://www.nice.org.uk/guidance/ta681. Accessed 21 Feb 2022.
Melo A, Carrascosa JM, Torres T. Baricitinib for the treatment of atopic dermatitis. J Dermatolog Treat. 2021. https://doi.org/10.1080/09546634.2021.1967268.
De Bruin-Weller M, Serra-Baldrich E, Barbarot S, et al. Indirect treatment comparison of baricitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis [abstract]. In: 16th EADV Symposium. 2021.
Reich K, Simpson E, Wollenberg A, et al. Efficacy with continuous dosing, down-titration, or treatment withdrawal after successful treatment with baricitinib in patients with moderate-to-severe atopic dermatitis [abstract no. 041]. J Invest Dermatol. 2021;141(10 Suppl 2):S155.
Akbarzadeh-Khiavi M, Torabi M, Rahbarnia L, et al. Baricitinib combination therapy: a narrative review of repurposed Janus kinase inhibitor against severe SARS-CoV-2 infection. Infection. 2021. https://doi.org/10.1007/s15010-021-01730-6.
Acknowledgements
During the peer review process, the manufacturer of baricitinib was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
Sheridan Hoy is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: N. Sideris, First Department of Dermatology, School of Medicine, Aristotle University, Thessaloniki, Greece; M. Sikora, National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, Poland.
The original online version of this article was revised due to open access retrospectively.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hoy, S.M. Baricitinib: A Review in Moderate to Severe Atopic Dermatitis. Am J Clin Dermatol 23, 409–420 (2022). https://doi.org/10.1007/s40257-022-00684-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-022-00684-1