- 1Department of Oncology, Hospital Israelita Albert Einstein, São Paulo, Brazil
- 2Medical Oncology Phase I Clinical Trials, HonorHealth Research Institute, Phoenix, AZ, United States
- 3Center for Personalized Medicine, Hospital Israelita Albert Einstein, São Paulo, Brazil
Although a relatively uncommon tumor, cholangiocarcinoma is on the rise globally. Of note, most patients are diagnosed with metastatic disease, and the prognosis is poor with cytotoxic chemotherapy. Strategies targeting specific genomic alterations have demonstrated promising activity in recent years and could represent a new therapeutic avenue for these patients. In this review, we will address the biology and clinical results of FGFR inhibition in intrahepatic cholangiocarcinoma, highlighting limitations associated with treatment and discussing the use of circulating tumor DNA to detect mechanisms of resistance.
Introduction
Biliary tract cancers (BTCs) are a group of heterogenous and rare malignancies that arise from any point of the biliary tract yet are uniformly associated with poor prognosis. BTCs are subdivided in intrahepatic cholangiocarcinoma (ICCA), extrahepatic cholangiocarcinoma (ECCA) and gallbladder cancer. ICCA originates from within the liver parenchyma, whereas ECCA can arise from any portion of the biliary tract outside of the liver, which can be further classified as hilar or distal cholangiocarcinoma (1). Incidence worldwide is increasing, both from ICCA and ECCA (2, 3). The estimated number of new cases of primary liver cancer, including hepatocellular carcinoma and biliary cancers, to have occurred globally in 2020, were of 906.000, of which ICCA accounts for approximately 10-15% (4).
The therapy of choice for advanced BTCs was established by the ABC-02 phase III trial, OS was significantly improved with gemcitabine and cisplatin versus gemcitabine (median 11.7 versus 8.1 months, HR 0.64) (5, 6). A phase II, non-randomized, single-arm clinical trial investigated the addition of nab-paclitaxel to gemcitabine-cisplatin (7). Median PFS was 12.2 months, and median OS was 19.2 months, which compares favorably to historical controls. Lately, positive results with the addition of durvalumab to chemotherapy was achieved in the TOPAZ-1 trial (8). In the study, durvalumab combined with cisplatin and gemcitabine conferred a 20% reduction in the risk of death compared with gemcitabine and cisplatin alone, meeting the primary endpoint of the trial, PFS and response rate were also improved with the combination (8). Although FOLFIRINOX is an effective regimen in pancreatic cancer, in advanced biliary cancers it was not superior to gemcitabine and cisplatin in the phase II randomized trial PRODIGE 38 AMEBICA (9). For second-line chemotherapy, results are less encouraging. Randomized trials identified mFOLFOX or 5FU plus liposomal irinotecan as regimens considered second-line options with improvements in OS for patients who have progressed after gemcitabine-based treatment, although more efficacious treatments are in need (10, 11).
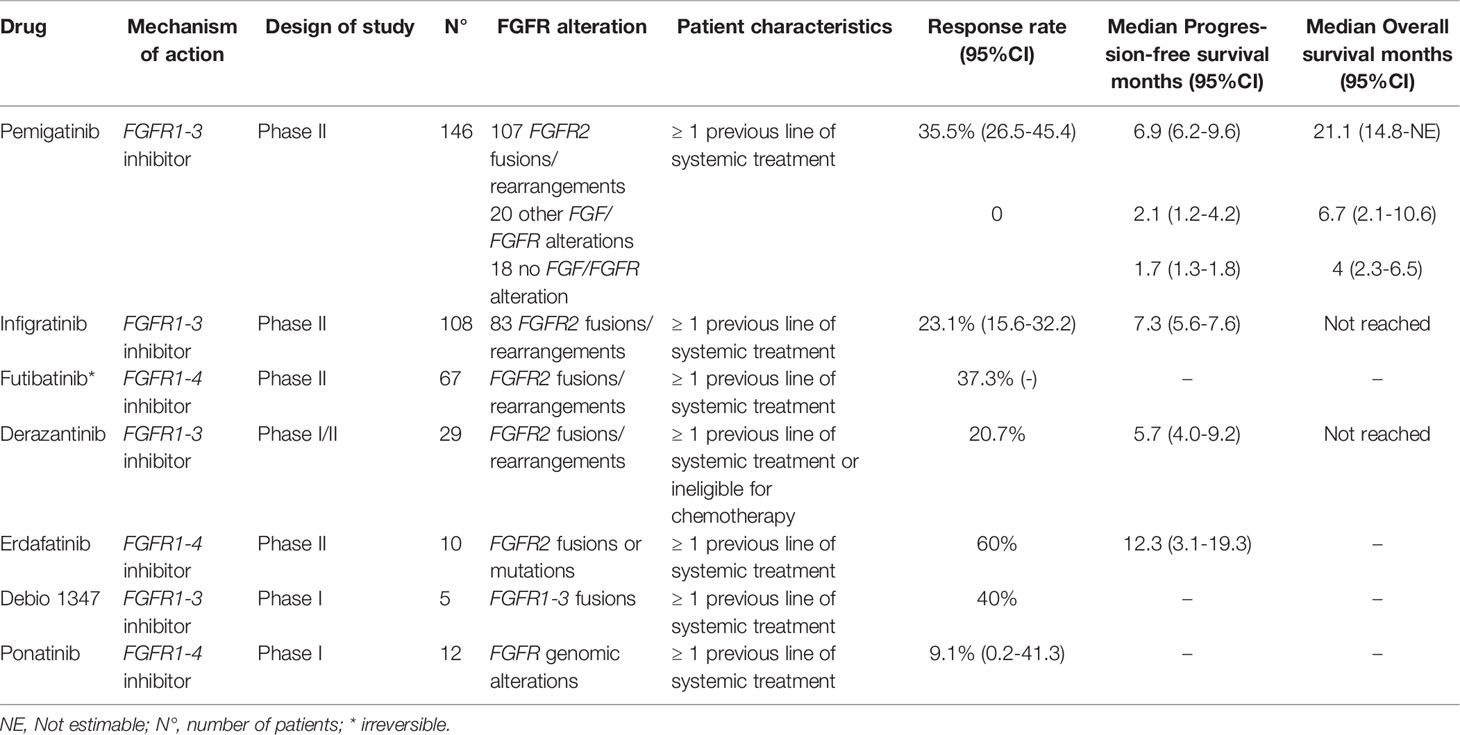
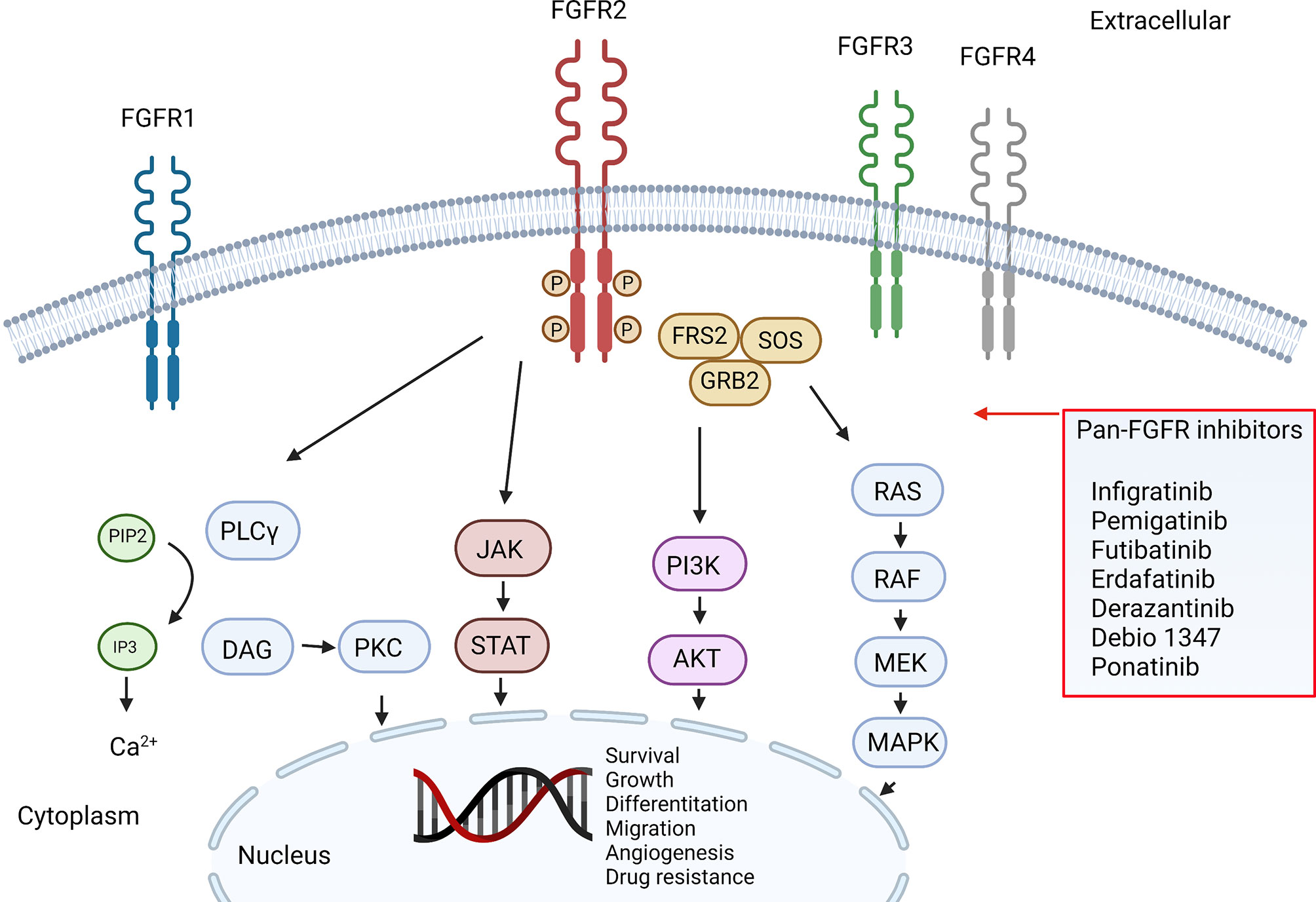
Biomarkers are present in varying patterns among ICCA and ECCA, and such differences highlight tumor-specific oncogenic pathways (12). Some of these biomarkers predicted the response to fibroblast growth factor receptor (FGFR) inhibitors, which target FGFR2-fusions in ICCA (Table 1, Figure 1). The FGFR2 belongs to the FGFR family of tyrosine kinases receptors. The family consists of 4 genes that encode single-pass transmembrane receptors that bind to FGF on the extracellular domain. Ligand binding trigger a signaling cascade that may exercise several cellular functions, including cell survival (13). It is estimated that FGFR2 genomic alterations are present in around 10-15% of ICCA, most of them consisting of fusions (14), but also different aberrations can drive oncogenic transformation, such as mutations and amplifications, which may account for up to 3% of the cases (15).
Chromosomal rearrangements (i.e., Fusions) cause intragenic translocations that encode functional proteins derived from each of the original proteins. FGFR2 partners up with other proteins with strong dimerization capacity, resulting in constitutive receptor activation and downstream signaling (16). Normally, FGF-FGFR signaling is triggered by the ligand-dependent receptor dimerization. The activation of the receptor leads to intracellular phosphorylation of receptor kinase domains, a cascade of intracellular signaling, and gene transcription. FGFR2 constitutive kinase activity is linked to oncogene addictive pathways including the RAS-MAPK, JAK-STAT, and PIK3-AKT-mTOR, promoting progressive growth, invasiveness, epithelial-mesenchymal transition and neo angiogenesis, Figure 1 (17). Single point mutations have been shown as well to increase FGFR activity by enhancing ligand binding of altering ligand specificity, they can also impair autoinhibitory brakes, which eventually turn to constitutive activity of the receptor kinase domain (18). All this alterations in FGFR genes, including activating mutations, chromosomal translocations, gene fusions, and gene amplifications, can result in ligand-independent signaling which increase receptor kinase activity.
Pemigatinib
Pemigatinib is a tyrosine multi-kinase inhibitor that blocks FGFR1-3, with weaker activity against FGFR4 [Merz, Valeria, Camilla Zecchetto, and Davide Melisi. “Pemigatinib, a potent inhibitor of FGFRs for the treatment of cholangiocarcinoma.” Future Oncology 17.4 (2020): 389-402]. It has been shown that pemigatinib inhibits the growth of tumor cell lines in pre-clinical models, suppressing growth of xenografted tumor models with FGFR alterations (19).
Initially, pemigatinib was evaluated in a phase I/II open-label study in a subset of patients with advanced solid tumors (FIGHT-101) (20). In this study, pemigatinib was evaluated in three subsets of patients. Groups 1 and 3 had unselected advanced solid tumors and group 2 tumors harboring FGF/FGFR alteration. All patients with advanced solid tumors were refractory to prior therapy and had no further effective standard therapy.
Patients received pemigatinib orally once daily on a 21-day cycle (2-weeks on/1-week off). In the dose escalation group 1, first 3 cohorts (1-4 mg once daily) evaluated single patients and subsequently a 3 + 3 design was used (6-20 mg once daily). In the dose expansion group 2, patients with FGFR rearrangements started on 9 mg once daily and increased to 13.5 mg once daily. In part 3 (dose-finding and expansion) pemigatinib could be used in together with standard systemic therapies. Overall, about half of the patients presented hyperphosphatemia and fatigue, other adverse events observed included dry mouth, alopecia and stomatitis; most frequent grade ≥3 adverse events were pneumonia (10%), fatigue (8%), and hyponatremia (8%). Hyperphosphatemia was easily managed with diet and phosphate binders; further dose modifications was also necessary. Based on preliminary safety and efficacy, the recommended phase II dose selected was 13.5 mg once daily. In the dose expansion cohort, group 2, four patients with cholangiocarcinoma were treated with pemigatinib, with one achieving a partial response (PR) taking 9mg daily, with duration of response still ongoing at data cut-off (20).
The efficacy of pemigatinib in cholangiocarcinoma harboring FGFR alterations was further evaluated in the phase II study FIGHT-202 (21). Patients with cholangiocarcinoma and disease progression after at least one previous treatment were assigned to one of three cohorts: patients with FGFR2-fusions or rearrangements, patients with other FGFR alterations, or patients with no FGFR alterations. The primary endpoint was objective response rate (ORR) among those with fusions or rearrangements. From 1206 patients prescreened, a total of 146 patients were enrolled from multiple centers in USA, Europe, Middle East, and Asia; 107 patients had cholangiocarcinoma harboring fusions or rearrangements, 20 harbored other FGF/FGFR alterations, and 18 had no FGF/FGFR alterations. All patients received at least one dose of pemigatinib. After a median follow-up of 17.8 months, an ORR of 35.5% was observed in the 107 patients with fusions or rearrangements. Median PFS was 6.9 months and median OS was 21.1 months. No patients with other FGF/FGFR alterations achieved responses. The most common all-grade adverse event was hyperphosphatemia in 60% patients. Most frequent grade ≥3 adverse events were hypophosphatemia, arthralgia, stomatitis, hyponatremia, abdominal pain, and fatigue. Most frequent serious adverse events were abdominal pain, pyrexia, cholangitis, and pleural effusion. There were no treatment related deaths (21). Additionally, in another study, genomic analysis of patients who progressed on pemigatinib also revealed important information about this treatment (15). No statistical difference was observed in RR and PFS between cases classified as FGFR2-fusion versus rearranged (15). However, patients with co-occurring tumor suppression gene loss (e.g., BAP1, CDKN2A/B, TP53, PBRM1, ARID1A, or PTEN) had shorter median PFS (p= 0.0003) (15).
Hyperphosphatemia is one of the most common adverse events related to FGFR inhibitors. It is an on-target effect related to FGFR inhibition [Kommalapati, Anuhya, et al. “FGFR inhibitors in oncology: insight on the management of toxicities in clinical practice.” Cancers 13.12 (2021): 2968]. Multiple strategies are proposed to manage or prevent this adverse event, which includes dietary modifications, phosphate-lowering therapies classified into phosphate binders and phosphaturic agents and dose or schedule modifications. Available phosphate binders include magnesium hydroxide, calcium and iron-based regimens, lanthanum carbonate, and sevelamer. A phosphaturic agent commonly used is acetazolamide [Kommalapati, Anuhya, et al. “FGFR inhibitors in oncology: insight on the management of toxicities in clinical practice.” Cancers 13.12 (2021): 2968].
On April 2020, the U.S. Food and Drug Administration (FDA) approved pemigatinib for the treatment of patients with previously treated advanced cholangiocarcinoma with FGFR2-fusion or rearrangement. Pemigatinib was also approved in the same terms by European Commission on March 2021. Currently, an international phase III randomized trial is recruiting patients to address pemigatinib against platinum-based chemotherapy as first-line therapy for unresectable or metastatic cholangiocarcinoma with FGFR2-fusions or rearrangements (22).
Infigratinib
The FGFR inhibitor (FGFRi) infigratinib was prospectively evaluated in patients with advanced cholangiocarcinoma with FGFR genomic alterations. In this phase II study, a total of 61 patients were evaluated and treated with infigratinib 125 mg orally for 21 days of each 28-day cycle until unacceptable toxicity or disease progression. All patients were previously treated with chemotherapy, including 67% of patients with at least two previous treatments. Most patients had cholangiocarcinoma harboring fusions, (n=48, 78.7%), eight patients had FGFR mutations, and three patients had amplifications (23). Eleven (18%) patients were treated previously with 3 lines of systemic therapy, and 19.7% with 4 lines. RR of patients harboring fusions was 18.8% [23. Overall DCR was 75.4% with a median PFS of 5.8 months. Updated results of the study were presented in a cohort of 108 patients, with 83 harboring FGFR2-fusions (24). In this subgroup, overall RR was 23.1%. A numerically higher RR was observed in patients treated in the second-line setting, RR of 34% (17/50), as compared with patients treated in the third or later-lines of systemic treatment (13.8%, 8/58). The median PFS of the cohort with fusions was 7.3 months. This result suggests that the efficacy of FGFR2 inhibitors may be higher in earlier lines of systemic treatment for advanced cholangiocarcinoma; ergo, studies evaluating these drugs on first-line setting might demonstrate higher benefit of these drugs (24). Common adverse events (any grade) included hyperphosphatemia in 76.9%, in more than half of patients were observed eye disorders and stomatitis, fatigue was also common, in about 40% of patients treated. It is important to state that in this study all patients received prophylaxis with the oral phosphate binder sevelamer. On May 2021, based on these results, the FDA granted accelerated approval for infigratinib for the treatment of patients with previously treated advanced cholangiocarcinoma with an FGFR2-fusion or rearrangement. A randomized phase III trial of infigratinib versus gemcitabine plus cisplatin chemotherapy as first-line therapy for unresectable or metastatic cholangiocarcinoma with FGFR2-fusions or rearrangements is underway (25).
Futibatinib
Futibatinib is an oral, highly selective, FGFR1-4 irreversible inhibitor, unlike other drugs of this class that work by competitive antagonism. Futibatinib has first been tested in humans in a phase I dose-escalation study (FOENIX-101) which included 86 highly pretreated patients with diverse advanced solid tumors harboring FGFR aberrations. The study identified the 20mg daily dose as the recommended phase 2 dose. Five patients (5,8%) had an objective response, in which 3 patients had ICCA with FGFR2-fusions (26). Futibatinib was further tested in a dose expansion phase on 45 patients with cholangiocarcinoma (41 with ICCA) harboring FGFR2-aberrations, 28 of them (62%) FGFR2-fusions and 17 (38%) other FGF-FGFR aberrations. All patients had an ECOG score of 0 or 1 and had received prior systemic therapy, including 13 patients who had received at least one reversible FGFRi. Of the 28 patients with FGFR2-fusions, seven achieved confirmed PR (25%) and 15 patients (54%) had stable disease (SD). Four confirmed PR occurred in patients previously treated with FGFRis, 3 of them with FGFR2-fusions (27). Grade 3 TRAE occurred in 41 patients (48%) of the overall population, the most frequent of them were hyperphosphatemia (12%), hyponatremia (7%) and anemia (6%). Other frequent any grade treatment emergent adverse events were diarrhea (37%), constipation (34%), dry mouth (29%), nausea (29%), and anemia (26%). Nail and ocular toxicity were also common.
That futibatinib achieves objective and durable responses after acquired secondary resistance to other FGFRis may be attributable to its mechanism of action of covalent irreversible binding, permanently deactivating FGFR2 enzymatic activity. Additional translational studies demonstrated futibatinib impressive capacity of overcoming diverse secondary FGFR2 kinase domain mutations, providing evidence of benefit for serial biopsies and/or ctDNA analysis after treatment failure to identify potential strategies to overcome treatment resistance (28).
Futibatinib was further tested in the phase II, open-label, multicenter FOENIX-CCA2 trial in patients with locally advanced or metastatic cholangiocarcinoma with FGFR2-fusions or other rearrangements who have progressive disease (PD) after at least 1 systemic line of therapy, with no prior use of inhibitors. Interim results have been presented for 67 patients who were followed for at least 6 months. Most (82%) had FGFR2-fusions, and 18% other rearrangements. The ORR was 37.3%, median duration of response of 8.3 months and disease control rate of 82% for the overall population (29). Additionally, the phase III, open-label, randomized, FOENIX-CCA3 trial is currently recruiting patients to evaluate futibatinib efficacy versus gemcitabine-cisplatin chemotherapy in the treatment of advanced or recurrent ICCA harboring FGFR2 gene rearrangements in the first-line setting (NCT04093362).
Derazantinib
Derazantinib is a multi-kinase competitive inhibitor with potent activity against FGFR1-3. It was first tested in a phase I trial of an unselected patient population with advanced solid tumors (30). The study defined the 300mg daily dose as the recommended phase 2 dose. In this population, there were ten patients with ICCA, of which five harbored FGFR2-fusions. Two of these patients showed partial and durable responses, while one patient showed SD. These results prompted enrolment to a second part of the phase 1/2 trial of patients with FGFR2-fusion positive metastatic or inoperable ICCA, who had either progressed after at least one line of treatment or were ineligible for chemotherapy. Six (20.7%) achieved PR, 18 (62.1%) SD and five patients (17.2%) had PD. The median duration of disease control on those patients who had either response or SD was 5.8 months and the median PFS was 5.7 months. Median OS was not reached after a median follow up of 20 months. Adverse events were common, and treatment discontinuation occurred with four patients because of upper gastrointestinal bleeding. Hyperphosphatemia was reported in 22 patients but required no dose adjustment or interruption. Eye toxicity occurred in 12 patients (41.4%) which demanded dose interruption and/or reduction in seven patients (24.1%) (31). These comprised data led to the currently enrolling FIDES-01 trial, a phase 2 open-label, single-arm trial testing 300mg daily of derazantinib for patients with ICCA that harbor FGFR2-fusions, but also mutations or amplifications, as it was recently shown that derazantinib has similar efficacy in these cases (32).
Erdafitinib
Erdafitinib is a potent, oral, FGFR1-4 tyrosine kinase competitive inhibitor. A four-step phase I clinical trial assessed erdafitinib’s safety and tolerability, first in an unselected patient population and subsequently in patients with FGFR alterations, such as FGFR3 mutations in urothelial carcinoma and FGFR2-fusions in ICCA (33). Although 187 patients were included, only 11 patients had ICCA, of which eight harbored FGFR2-fusions and three with FGFR mutations. In the overall cholangiocarcinoma population, 3 of 11 (27%) patients had PR, with a median duration of response of 11,4 months. The most common adverse events were hyperphosphatemia (64%), dry mouth (42%), and stomatitis, most of them of grade 1/2 severity. Skin changes, nail disorders, and eye disorders were also common. Grade 3 events or higher were infrequent, the main one reported was anemia in 17 patients (9%). Adverse events were considered the main cause of death in 9 patients (5%), including two cases of bleeding complications. Nevertheless, adverse events were mostly mild and manageable.
Erdafitinib is under further investigation in the LUC2001 trial, a phase II multi-center, open-label, clinical trial. Preliminary results of an Asian cohort of this study have been presented (34). Thirty-four patients with cholangiocarcinoma were found to harbor FGFR gene alterations and 14 of them were evaluated and treated with erdafitinib 8mg daily, with possible dose increases. Of these 14 patients, 8 had FGFR2-fusion ICCA while the remaining patients had different alterations and all patients had been previously treated with chemotherapy. In 10 evaluable patients with FGFR2 alterations (gene fusion or mutation), there were 6 (60%) confirmed PR and 4 (40%) SD. Median PFS was 12.35 months. Safety and tolerability data were like those previously reported and did not differ in the Asian population compared to other ethnic groups.
Debio-1347
Debio-1347 is an oral highly selective ATP competitive FGFR1-3 inhibitor. The first-in-human study with the compound selected 58 patients with FGFR1-3 alterations and defined 80mg daily as the standard dose (35). Efficacy was encouraging and safety analysis showed a manageable toxicity profile, with no deaths related to treatment. In the dose expansion phase, of the 18 patients enrolled for evaluable response, 5 had cholangiocarcinoma, of which 4 harbored FGFR2-fusion and one with FGFR1-fusion, being the only one who had PD after treatment. The other 4 had controlled disease, two with PR and two with SD. In total, 3 of the 18 patients had objective responses, with median duration of response of 16.1 weeks (range: 8.4-22.8) and median PFS of 18,3 weeks (36). The FUZE phase II basket-trial will further evaluate debio-1347 efficacy and tolerability in patients with solid tumors harboring FGFR1-3 gene fusions previously treated and recruitment has already been completed (NCT03834220).
Ponatinib
Ponatinib is a FGFR1-4 tyrosine kinase inhibitor, along with inhibition effect in several other kinases including KIT, RET, SRC, VEGFR and PDGFR (37). A pilot study evaluating ponatinib in biliary tract cancers refractory to systemic treatments and FGFR alterations was early terminated after interim analysis (38). Overall disease control rate was 45.5% however objectives responses was observed in just one from eleven patients treated and assessed. Considering the modest activity of this agent futures studies should evaluate combinations with other molecules or refining patient selection (38).
Discussion
FGFR2-fusions are an important target in cholangiocarcinoma to date; however, after an initially higher RR to these agents, most tumors will develop disease progression. Efforts are under way to identify mechanisms of resistance to this agents (15, 28, 39–41). Tumor resistance to FGFRis is identified in multiple tumor types and are mostly related to activation of different signaling pathways including MET, Eph3B, ERBB2/3 or EGFR and/or activation of intracellular signaling pathways without tyrosine kinase receptor dependence (42). Another observed factor of resistance with chronic exposure to FGFRis is induced epithelial-mesenquimal transition (39). In cholangiocarcinoma, gatekeeper mutations that modify the binding site of FGFRis and maintain activation of the FGF pathway have been described (39).
A sample from a patient with advanced FGFR2 fused cholangiocarcinoma, harboring FGFR2-CLIP1 fusion, was evaluated after progression to pemigatinib (40). Sanger sequencing on tumor samples after progression confirmed FGFR2-CLIP1 fusion in all samples, furthermore, whole-exome sequencing revealed 242 unique mutations to post progression and a FGFR2 kinase domain acquired mutation, FGFR2 N549H in a single liver tumor. The FGFR2 N549H mutation results in ligand-independent constitutive activation (40).
Analysis with circulating tumor DNA (ctDNA) is also a strategy to identify acquired mutations (40). ctDNA analysis in three patients with advanced cholangiocarcinoma who disease progressed after treatment with infigratinib, identified multiple mutations on FGFR kinase domain including N549H, N549K, V564F, E565A, K659M, L617V and K641R. Paired tissue analysis detected PI3K/PTEN pathway mutations in some samples (15, 41). In the study evaluating clinicogenomic analysis of pemigatinib-treated patients, they identified a total of 63 FGFR2 rearrangement partners genes (15). The most common fusion partners included BICC1 (27.9%), KIAA1217 (3.6%), TACC2 (2.9%), CCDC6 (2.9%), and AHCYL1 (2.9%). The second most frequent FGFR2 rearrangement identified was FGFR2-N/A (9.3%). N/A refers to rearrangements that occur in FGFR2 intron 17 or exon 18 fused to an intergenic region (15). Interestingly, no differences in RR and PFS were observed between different fusion partners (15). Eight patients that initially responded to pemigatinib and had disease progression were evaluated with genomic profiling in tissue or plasma (15). The evaluation showed that all patients presented at least one acquired FGFR2 mutation, suggesting that mechanisms of resistance to FGFRis are similar between the different available drugs (15).
Targeted sequencing of tumor DNA after progression could delineate combination strategies to overcome resistance. Upregulation of the PI3K/AKT/mTOR signaling pathway was observed in cell lines expressing acquired FGFR2 p.E565A after infigratinib treatment. Further exposure to mTOR inhibitors re-sensitized these cells to FGFR inhibition [Krook, Melanie A., et al. “Efficacy of FGFR inhibitors and combination therapies for acquired resistance in FGFR2-fusion cholangiocarcinoma.” Molecular Cancer Therapeutics 19.3 (2020): 847-857]. Another strategy to overcome resistance would be combined EGFR/FGFR inhibition. In a study evaluating patient-derived models of FGFR2-fusion-positive cholangiocarcinoma, inhibition of EGFR potentiated responses to FGFR inhibitors, durably suppressing MEK/ERK and mTOR signaling, increasing apoptosis, and causing marked tumor regressions in vivo. [Wu, Qibiao, et al. “EGFR inhibition potentiates FGFR inhibitor therapy and overcomes resistance in FGFR2 fusion-positive cholangiocarcinomaCombination Treatment in FGFR2-Fusion Cholangiocarcinoma.” Cancer Discovery.]
In conclusion, incorporation of circulating tumor DNA analysis or tissue genomic analysis after exposure and disease progression on FGFRis have the potential to characterize and understand specific gatekeeper mutations related to resistance and treatment failure. Exploring co-occurring mutations will be also necessary, considering that they could influence the effectiveness on these targeted treatments.
Author Contributions
MZ performed the writing, prepared the table. RP and GB in writing and reviewing. PU provided the input in writing and reviewing the paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Figure 1 was created with BioRender.com.
References
1. National Comprehensive Cancer Network. Hepatobiliary Cancers (Version 5.2021) (2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (Accessed December 6, 2021).
2. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncol (2016) 21:594–9. doi: 10.1634/theoncologist.2015-0446
3. Florio AA, Ferlay J, Znaor A, Ruggieri D, Alvarez CS, Laversanne M, et al. Global Trends in Intrahepatic and Extrahepatic Cholangiocarcinoma Incidence From 1993 to 2012. Cancer (2020) 126:2666–78. doi: 10.1002/cncr.32803
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
5. Uson PLS, Bogenberger J, Borad MJ. Advances in the Treatment of Biliary Tract Cancers. Curr Opin Gastroenterol (2020) 36:85–9. doi: 10.1097/MOG.0000000000000606
6. Weigt J, Malfertheiner P. Cisplatin Plus Gemcitabine Versus Gemcitabine for Biliary Tract Cancer. Expert Rev Gastroenterol Hepatol (2010) 4:395–7. doi: 10.1586/egh.10.45
7. Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, Cisplatin, and Nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol (2019) 5:824–30. doi: 10.1001/jamaoncol.2019.0270
8. Oh D-Y, He AR, Qin S, Chen L-T, Okusaka T, Vogel A, et al. A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study of Durvalumab in Combination With Gemcitabine Plus Cisplatin (GemCis) in Patients (Pts) With Advanced Biliary Tract Cancer (BTC): TOPAZ-1. J Clin Oncol (2022) 40:378–8. doi: 10.1200/JCO.2022.40.4_suppl.378
9. Phelip JM, Desrame J, Edeline J, Barbier E, Terrebonne E, Michel P, et al. Modified FOLFIRINOX Versus CISGEM Chemotherapy for Patients With Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J Clin Oncol (2022) 40.3:262–71. doi: 10.1200/JCO.21.00679
10. Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-Line FOLFOX Chemotherapy Versus Active Symptom Control for Advanced Biliary Tract Cancer (ABC-06): A Phase 3, Open-Label, Randomised, Controlled Trial. Lancet Oncol (2021) 22:690–701. doi: 10.1016/S1470-2045(21)00027-9
11. Yoo C, Kim K-P, Kim I, Kang MJ, Cheon J, Kang BW, et al. Liposomal Irinotecan (Nal-IRI) in Combination With Fluorouracil (5-FU) and Leucovorin (LV) for Patients With Metastatic Biliary Tract Cancer (BTC) After Progression on Gemcitabine Plus Cisplatin (GemCis): Multicenter Comparative Randomized Phase 2b Study. J Clin Oncol (2021) 39:4006–6. doi: 10.1200/JCO.2021.39.15_suppl.4006
12. Labib PL, Goodchild G, Pereira SP. Molecular Pathogenesis of Cholangiocarcinoma. BMC Cancer (2019) 19:185. doi: 10.1186/s12885-019-5391-0
13. Haugsten EM, Wiedlocha A, Olsnes S, Wesche J. Roles of Fibroblast Growth Factor Receptors in Carcinogenesis. Mol Cancer Res (2010) 8:1439–52. doi: 10.1158/1541-7786.MCR-10-0168
14. Ross JS, Wang K, Gay L, Al-Rohil R, Rand JV, Jones DM, et al. New Routes to Targeted Therapy of Intrahepatic Cholangiocarcinomas Revealed by Next-Generation Sequencing. Oncol (2014) 19:235–42. doi: 10.1634/theoncologist.2013-0352
15. Silverman IM, Hollebecque A, Friboulet L, Owens S, Newton RC, Zhen H, et al. Clinicogenomic Analysis of FGFR2-Rearranged Cholangiocarcinoma Identifies Correlates of Response and Mechanisms of Resistance to Pemigatinib. Cancer Discovery (2021) 11:326–39. doi: 10.1158/2159-8290.CD-20-0766
16. Li F, Peiris MN, Donoghue DJ. Functions of FGFR2 Corrupted by Translocations in Intrahepatic Cholangiocarcinoma. Cytokine Growth Factor Rev (2020) 52:56–67. doi: 10.1016/j.cytogfr.2019.12.005
17. Goyal L, Kongpetch S, Crolley VE, Bridgewater J. Targeting FGFR Inhibition in Cholangiocarcinoma. Cancer Treat Rev (2021) 95:102170. doi: 10.1016/j.ctrv.2021.102170
18. Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin Cancer Res (2016) 22:259–67. doi: 10.1158/1078-0432.CCR-14-3212
19. Liu PCC, Koblish H, Wu L, Bowman K, Diamond S, DiMatteo D, et al. INCB054828 (Pemigatinib), A Potent and Selective Inhibitor of Fibroblast Growth Factor Receptors 1, 2, and 3, Displays Activity Against Genetically Defined Tumor Models. PloS One (2020) 15:e0231877. doi: 10.1371/journal.pone.0231877
20. Saleh M, Gutierrez ME, Subbiah V, Smith DC, Asatiani E, Lihou CF, et al. Abstract CT111: Preliminary Results From a Phase 1/2 Study of INCB054828, a Highly Selective Fibroblast Growth Factor Receptor (FGFR) Inhibitor, in Patients With Advanced Malignancies. Cancer Res (2017) 77:CT111–1. doi: 10.1158/1538-7445.AM2017-CT111
21. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for Previously Treated, Locally Advanced or Metastatic Cholangiocarcinoma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol (2020) 21:671–84. doi: 10.1016/S1470-2045(20)30109-1
22. Bekaii-Saab TS, Valle JW, van Cutsem E, Rimassa L, Furuse J, Ioka T, et al. FIGHT-302: First-Line Pemigatinib vs Gemcitabine Plus Cisplatin for Advanced Cholangiocarcinoma With FGFR2 Rearrangements. Future Oncol (2020) 16:2385–99. doi: 10.2217/fon-2020-0429
23. Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol (2018) 36:276–82. doi: 10.1200/JCO.2017.75.5009
24. Javle MM, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla T, Waldschmidt DT, et al. Final Results From a Phase II Study of Infigratinib (BGJ398), an FGFR-Selective Tyrosine Kinase Inhibitor, in Patients With Previously Treated Advanced Cholangiocarcinoma Harboring an FGFR2 Gene Fusion or Rearrangement. J Clin Oncol (2021) 39:265–5. doi: 10.1200/JCO.2021.39.3_suppl.265
25. Javle MM, Borbath I, Clarke SJ, Hitre E, Louvet C, Mercade TM, et al. Infigratinib Versus Gemcitabine Plus Cisplatin Multicenter, Open-Label, Randomized, Phase 3 Study in Patients With Advanced Cholangiocarcinoma With FGFR2 Gene Fusions/Translocations: The PROOF Trial. J Clin Oncol (2019) 37:TPS4155–TPS4155. doi: 10.1200/JCO.2019.37.15_suppl.TPS4155
26. Bahleda R, Meric-Bernstam F, Goyal L, Tran B, He Y, Yamamiya I, et al. First-in-Human Study of Futibatinib, a Highly Selective, Irreversible FGFR1–4 Inhibitor in Patients With Advanced Solid Tumors. Ann Oncol (2020) 31:1405–12. doi: 10.1016/j.annonc.2020.06.018
27. Meric-Bernstam F, Arkenau H, Tran B, Bahleda R, Kelley R, Hierro C, et al. Efficacy of TAS-120, an Irreversible Fibroblast Growth Factor Receptor (FGFR) Inhibitor, in Cholangiocarcinoma Patients With FGFR Pathway Alterations Who Were Previously Treated With Chemotherapy and Other FGFRis. Ann Oncol (2018) 29:v100. doi: 10.1093/annonc/mdy149
28. Goyal L, Shi L, Liu LY, de la Cruz FF, Lennerz JK, Raghavan S, et al. TAS-120 Overcomes Resistance to Atp-Competitive FGFRis in Patients With FGFR2 Fusion–Positive Intrahepatic Cholangiocarcinoma. Cancer Discovery (2019) 9:1064–79. doi: 10.1158/2159-8290.CD-19-0182
29. Bridgewater J, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic T, et al. 54p Efficacy and Safety of Futibatinib in Intrahepatic Cholangiocarcinoma (iCCA) Harboring FGFR2 Fusions/Other Rearrangements: Subgroup Analyses of a Phase II Study (FOENIX-Cca2). Ann Oncol (2020) 31:S261–2. doi: 10.1016/j.annonc.2020.08.032
30. Papadopoulos KP, El-Rayes BF, Tolcher AW, Patnaik A, Rasco DW, Harvey RD, et al. A Phase 1 Study of ARQ 087, an Oral Pan-FGFRi in Patients With Advanced Solid Tumours. Br J Cancer (2017) 117:1592–9. doi: 10.1038/bjc.2017.330
31. Mazzaferro V, El-Rayes BF, Droz dit Busset M, Cotsoglou C, Harris WP, Damjanov N, et al. Derazantinib (ARQ 087) in Advanced or Inoperable FGFR2 Gene Fusion-Positive Intrahepatic Cholangiocarcinoma. Br J Cancer (2019) 120:165–71. doi: 10.1038/s41416-018-0334-0
32. Droz Dit Busset M, Shaib WL, Harris WP, Damjanov N, Borad M, Vogel A, et al. 45p Efficacy of Derazantinib in Intrahepatic Cholangiocarcinoma Patients With FGFR2 Mutations or Amplifications: Pooled Analysis of Clinical Trials and Early Access Programs. Ann Oncol (2020) 31:S1231. doi: 10.1016/j.annonc.2020.08.2204
33. Bahleda R, Italiano A, Hierro C, Mita A, Cervantes A, Chan N, et al. Multicenter Phase I Study of Erdafitinib (JNJ-42756493), Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients With Advanced or Refractory Solid Tumors. Clin Cancer Res (2019) 25:4888–97. doi: 10.1158/1078-0432.CCR-18-3334
34. Park JO, Feng Y-H, Chen Y-Y, Su W-C, Oh D-Y, Shen L, et al. Updated Results of a Phase IIa Study to Evaluate the Clinical Efficacy and Safety of Erdafitinib in Asian Advanced Cholangiocarcinoma (CCA) Patients With FGFR Alterations. J Clin Oncol (2019) 37:4117–7. doi: 10.1200/jco.2019.37.15_suppl.4117
35. Voss MH, Hierro C, Heist RS, Cleary JM, Meric-Bernstam F, Tabernero J, et al. Open-Label, Multicenter, Dose-Escalation Study of the Oral Selective FGFRi Debio 1347 in Patients With Advanced Solid Tumors Harboring FGFR Gene Alterations. Clin Cancer Res (2019) 25:2699–707. doi: 10.1158/1078-0432.CCR-18-1959
36. Cleary JM, Iyer G, Oh D-Y, Mellinghoff IK, Goyal L, Ng MCH, et al. Final Results From the Phase I Study Expansion Cohort of the Selective FGFRi Debio 1,347 in Patients With Solid Tumors Harboring an FGFR Gene Fusion. J Clin Oncol (2020) 38:3603–3. doi: 10.1200/jco.2020.38.15_suppl.3603
37. Gozgit JM, Wong MJ, Moran L, Wardwell S, Mohemmad QK, Narasimhan NI, et al. Ponatinib (AP24534), a Multitargeted Pan-FGFRi With Activity in Multiple FGFR-Amplified or Mutated Cancer Models. Mol Cancer Ther (2012) 11:690–9. doi: 10.1158/1535-7163.MCT-11-0450
38. Ahn DH, Uson Junior PLS, Masci P, Kosiorek H, Halfdanarson TR, Mody K, et al. A Pilot Study of Pan-FGFRi Ponatinib in Patients With FGFR-Altered Advanced Cholangiocarcinoma. Invest New Drugs (2021) 40:134–41. doi: 10.1007/s10637-021-01170-x
39. Lau DK, Jenkins L, Weickhardt A. Mechanisms of Acquired Resistance to Fibroblast Growth Factor Receptor Targeted Therapy. Cancer Drug Resist (2019) 2:568–79. doi: 10.20517/cdr.2019.42
40. Krook MA, Bonneville R, Chen H-Z, Reeser JW, Wing MR, Martin DM, et al. Tumor Heterogeneity and Acquired Drug Resistance in FGFR2-Fusion-Positive Cholangiocarcinoma Through Rapid Research Autopsy. Mol Case Stud (2019) 5:a004002. doi: 10.1101/mcs.a004002
41. Goyal L, Saha SK, Liu LY, Siravegna G, Leshchiner I, Ahronian LG, et al. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients With FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discovery (2017) 7:252–63. doi: 10.1158/2159-8290.CD-16-1000
Keywords: cholangiocarcinoma, FGFR2, fusions, precision medicine, pemigatinib
Citation: Zugman M, Botrus G, Pestana RC and Uson Junior PLS (2022) Precision Medicine Targeting FGFR2 Genomic Alterations in Advanced Cholangiocarcinoma: Current State and Future Perspectives. Front. Oncol. 12:860453. doi: 10.3389/fonc.2022.860453
Received: 23 January 2022; Accepted: 14 March 2022;
Published: 04 April 2022.
Edited by:
Nadia M. Hamdy, Ain Shams University, EgyptReviewed by:
Alberto Servetto, University of Naples Federico II, ItalyValeria Merz, University of Verona, Italy
Copyright © 2022 Zugman, Botrus, Pestana and Uson Junior. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pedro Luiz Serrano Uson Junior, pedro.serrano@einstein.br
 Miguel Zugman
Miguel Zugman Gehan Botrus
Gehan Botrus Roberto Carmagnani Pestana1
Roberto Carmagnani Pestana1 Pedro Luiz Serrano Uson Junior
Pedro Luiz Serrano Uson Junior