- Department of Hematology and Medical Oncology, Winship Cancer Institute of Emory University, Atlanta, GA, United States
Hepatocellular carcinoma (HCC) is a serious global health problem as one of the leading causes of cancer-related death worldwide. Systemic therapy for advanced HCC has progressed with the development of molecular targeted agents, however survival benefits remain modest. More recently, immune checkpoint inhibitors (ICI) have emerged and exhibited promising therapeutic benefits in a subset of patients. Physiologically, the intrinsic microenvironment in the liver is immunosuppressive, which represents a major obstacle for effective immune therapies in primary and secondary liver malignancies. For this reason, combination therapies that can overcome immune inhibitory mechanisms and enhance the immune response are a rationale approach for drug development in HCC. A recent example is the combination of the anti-PD-L1 antibody (atezolizumab) and anti-VEGF-A antibody (bevacizumab), which has shown significant improvement in survival as compared to standard of care in the first-line treatment for HCC. Other immunotherapy approaches including cancer vaccines and adoptive cell therapy are also under investigation. This review summarizes the key trials leading to our current HCC treatment options and provides an overview of future immune-based strategies in development.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and fourth leading cause of cancer mortality worldwide with approximately 800,000 deaths per year. Although the incidence rates of most malignancies are declining, the incidence rate of HCC continues to increase. It is estimated that over one million individuals will develop HCC annually by 2025 (1, 2). The majority of HCC cases (>70%) occur in Asia, however numbers in the Western world are rising. Chronic liver disease due to Hepatitis B (HBV) and C (HCV) viruses are the most common causes of HCC, followed by other etiologies, including excess alcohol intake, non-alcoholic fatty liver disease (NAFLD) associated with metabolic syndrome, and environmental toxins (2, 3). The prognosis of HCC is largely determined by the stage at diagnosis. Potentially curative treatment options including surgical resection and liver transplantation are offered with earlier stages of disease and result in over 70% 5-year survival rates (4). Unfortunately, the majority of patients present with advanced stage HCC, which has dismal long-term survival rates. Clinical challenges in the management of advanced stage HCC include the underlying medical liver disease, altered liver functions, and systemic effects of liver dysfunction which can complicate side effects of commonly used therapies (4, 5).
Prior to the advent of sorafenib, systemic therapy for advanced stage HCC was limited to cytotoxic agents (doxorubicin), which historically have shown poor response rates (< 25%) and significant toxicities (6). Over the past decade, there has been significant advancement in HCC treatment with the development of molecular targeted agents and immune therapies. Sorafenib was the first multi-targeted tyrosine kinase inhibitor (TKI) to demonstrate a survival benefit in advanced HCC. Later studies demonstrated clinical benefits from other TKIs, including lenvatinib (7), cabozantinib (8), and regorafenib (9). Although the advent of TKIs was a major breakthrough in HCC treatment, the prognosis remained poor with a median overall survival of 10-14 months, highlighting the unmet need to develop novel therapies to further improve patient survival outcomes (7–9).
In recent years, immunotherapies have rapidly changed the scope of cancer treatment with the growing recognition that immune evasion is an important mechanism of cancer progression (10). The effectiveness of immunotherapy demonstrated in other cancers like melanoma led to studies evaluating its use in HCC (11). Immune checkpoint inhibitors (ICI), including atezolizumab combined with bevacizumab, pembrolizumab, and nivolumab combined with ipilimumab, have since been approved for treatment of advanced HCC and are now incorporated into current HCC treatment guidelines (12–15). In this review, we will provide an update about the current landscape of the systemic therapies in advanced HCC (Table 1) and discuss novel strategies on the horizon in the era of immuno-oncology.
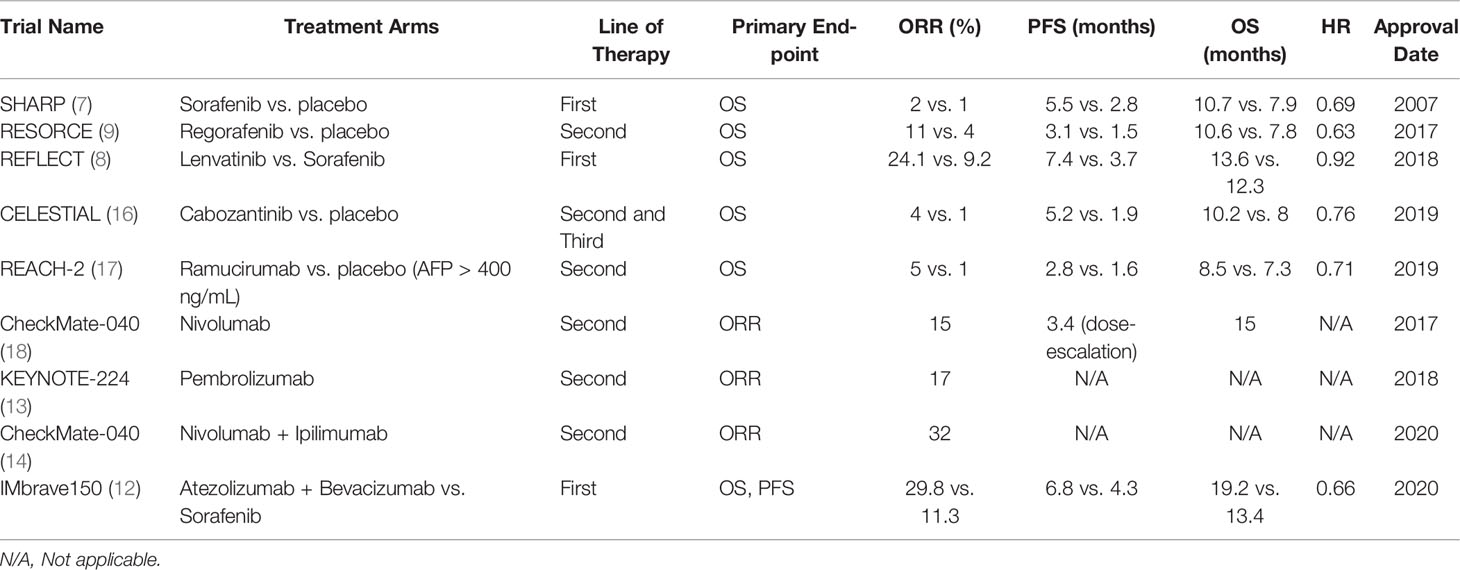
Table 1 Key findings of landmark clinical trials for the approved systemic therapies in advanced HCC.
Liver Tolerogenicity and HCC Immune Evasion
The liver has a unique immunosuppressive microenvironment that prevents overactivation of the immune system from constant exposure to antigens arising from the gut (19). Liver sinusoidal endothelial cells (LSECs) form the fenestrated barrier between the liver parenchyma and sinusoids, and act as antigen-presenting cells (APCs). However, LSECs express high levels of immunosuppressive receptors like program death receptor ligand 1 (PD-L1) and low levels of costimulatory molecules CD80 and CD86, decreasing their ability to activate T cells. This is largely due to their role of limiting immune responses to gut bacterial molecules in order to avoid unnecessary inflammatory tissue damage (19, 20). Kupffer cells (KCs) are the liver-residing macrophages with the primary function of pathogen clearance (21). Similar to LSECs, KCs induce tolerance by low expression of major histocompatibility complex (MHC) molecules, secretion of immunosuppressive cytokines like IL-10 and prostaglandins, and direct activation of inhibitory forkhead box P3 (FoxP3) that leads to expansion of regulatory T cells (Tregs) (22). Hepatic dendritic cells (DCs) also express low levels of co-stimulatory molecules and MHC as well as produce anti-inflammatory prostaglandins (23). Overall, this immunosuppressive microenvironment is necessary to maintain self-tolerance in the liver, although it poses a challenge to the development of anti-tumor immunity in HCC.
HCC development and progression are typically characterized by chronic inflammation of the liver caused by infection, toxins, and steatosis (24). Within this inflammatory state, multiple complex immunosuppressive mechanisms become activated in HCC and contribute to immune evasion. In HCC, immunosuppressive cytokines like IL-10 and TGF-β are continuously expressed and immune cells including Tregs, myeloid-derived suppressor cells (MDSCs), and M2 tumor-associated macrophages (TAM), accumulate in the liver (25, 26). MDSCs are immature myeloid cells that inhibit effector T cell function, increase expansion of Tregs, and upregulate PD-L1 (27). The increased interaction between programmed cell death protein (PD-1) on T cells and PD-L1 on cancer cells promotes T cell exhaustion or dysregulation, and higher numbers of PD-1+ CD8+ T cells have been associated with disease progression and poorer prognosis (28–30). Defects in APC molecules also occur in HCC, resulting in downregulation of HLA-1 expression and thus ineffective presentation of tumor antigens that allows for escape from cytotoxic T cells (31). Together, these mechanisms represent potential treatment strategies in order to enhance anti-tumor immunity in HCC, some of which have been successfully utilized to develop FDA-approved agents and others that are currently under investigation.
Current Treatment Options
Molecular Targeted Agents
Sorafenib
Sorafenib inhibits the activity of multiple tyrosine kinases involved in tumor angiogenesis and proliferation, including vascular endothelial growth factor receptors (VEGFR) 1-3, Raf kinases, and platelet-derived growth factor receptor beta (PDGFR-β). The landmark phase III SHARP trial randomized 602 previously untreated patients, predominantly from a Western population, with advanced HCC to receive either sorafenib (400 mg twice daily) or placebo. Median overall survival (OS) was significantly better in the sorafenib arm at 10.7 months versus 7.9 months in the placebo group (Hazard ratio (HR) 0.69, p<0.001) (7). Similar positive results were reported in a parallel Asia-Pacific cohort (median OS 6.5 months for sorafenib vs. 4.2 months for placebo; HR 0.68, p=0.014). A potential explanation for the survival differences between the trials is the higher risk baseline tumor and patient characteristics in the Asia-Pacific study, including more patients with extrahepatic spread, higher alpha-fetoprotein (AFP) levels, and poorer ECOG performance status. Both studies notably restricted enrollment to patients with Child-Pugh A disease and reported similar treatment-related adverse events including hand-foot syndrome and diarrhea (7, 32). The GIDEON study observed over 3,000 HCC patients treated with sorafenib and found comparable safety profiles among Child-Pugh class A and B patients, suggesting that treatment may be used in higher degrees of liver dysfunction. However, the results should be interpreted with caution given the observational nature of the study, and treatment should be carefully assessed in those with more clinically significant underlying liver disease (33).
Regorafenib
Regorafenib inhibits multiple TKIs, including VEGFR 1-3, KIT, PDGFR-β, fibroblast growth factor receptors (FGFR) 1-2, BRAF and RET. The phase III RESORCE trial randomized 573 patients who progressed on sorafenib to either regorafenib (160 mg once daily for three weeks on and one week off) or placebo. Regorafenib resulted in significant prolongation in median OS (10.6 vs. 7.8 months, HR 0.63, p<0.0001) as well as median time to progression (TTP) (3.2 vs. 1.5 months) and objective response rates (ORR) (11% vs. 4%). Side effects of regorafenib were similar to those reported for sorafenib, including hand-foot syndrome, hypertension, and fatigue (9). Nearly one decade after sorafenib approval, regorafenib was the next TKI approved as second-line therapy for HCC (34).
Lenvatinib
Following sorafenib approval, multiple randomized phase III trials evaluating other drugs (sunitinib, brivanib, linifanib, erlotinib plus sorafenib) for first-line treatment failed (35–39). Lenvatinib is a multikinase inhibitor with activity against VEGFR 1-3, PDGFR-α, FGFR 1-4, KIT and RET. Based on promising results from a phase II study, the phase III non-inferiority REFLECT trial randomized 954 patients with unresectable HCC and Child-Pugh A disease to lenvatinib (12 mg/day for bodyweight ≥60 kg or 8 mg/day for bodyweight <60kg) or sorafenib (400 mg twice daily) in the first-line setting (8, 40). Lenvatinib was noninferior to sorafenib for the primary endpoint of median OS (13.6 vs. 12.3 months; HR 0.92). Lenvatinib also led to significantly higher progression-free survival (PFS) (7.4 vs. 3.7 months; HR 0.66, p<0.0001), median TTP (8.9 vs. 3.7 months; HR 0.63, p<0.0001), and ORR (24.1% vs. 9.2%; odds ratio (OR) 3.13, p<0.0001) compared to the control arm. Hypertension was more common for lenvatinib and hand-foot skin reaction for sorafenib, but toxicity profiles were overall relatively similar (8). Moreover, an exploratory analysis of the REFLECT trial demonstrated that objective response was an independent predictor of OS in patients with advanced HCC (median OS 22.4 months for responders vs. 11.4 months for nonresponders) (41).
Cabozantinib
The effectiveness of cabozantinib, a potent inhibitor of VEGFR1-3, MET, and AXL, in previously treated advanced HCC was shown in the phase III CELESTIAL trial. The study enrolled 707 patients with advanced HCC with up to two prior lines of treatment and assigned them to either cabozantinib (60 mg daily) or placebo. Cabozantinib achieved superior survival outcomes compared to placebo, with median OS 10.2 months versus 8 months (HR 0.76, p=0.005), median PFS 5.2 months versus 1.9 months (HR 0.44, p<0.001), and ORR 4% versus <1% (16). Survival benefit with cabozantinib was seen across high (≥400 ng/mL) and low (<400 ng/mL) baseline AFP levels, and resulted in more AFP responses (≥20% decrease from baseline at Week 8) that was associated with longer OS and PFS (42). Side effects of cabozantinib were similar to those of sorafenib, including hand-foot syndrome and hypertension (16).
Ramucirumab
Ramucirumab is a recombinant IgG1 monoclonal antibody (mAb) that inhibits VEGFR-2. A phase II study of 42 patients showed promising results of ramucirumab in the first-line setting, but did not make direct comparisons with sorafenib (43). Subsequently, the phase III REACH trial randomized 565 HCC patients after progression on sorafenib to ramucirumab (8 mg/kg every 2 weeks) or placebo, which showed no significant differences in median OS (9.2 months for ramucirumab vs. 7.6 months placebo, HR 0.87, p=0.14). However, a subgroup analysis indicated a significant survival benefit for patients with elevated AFP levels of >400 ng/mL (median OS for ramucirumab 7.8 months vs. 4.2 months placebo; HR 0.67, p=0.006) (44). The benefits seen in this particular subset of patients were validated by the REACH-2 study, which demonstrated an improved median OS of 8.5 months with ramucirumab compared to 7.3 months in the placebo arm (HR 0.71, p=0.019), and PFS was 2.8 months vs. 1.6 months (HR 0.45, p<0.0001) (17, 44). These findings could potentially be explained by the association between elevated AFP and increased angiogenesis, resulting in increased sensitivity to VEGFR-2 inhibition. Ramucirumab was generally well-tolerated, with peripheral edema and ascites reported as the most frequent treatment-related adverse events, and has the advantage of no associated hand-foot skin reaction (44).
Immunotherapy
Nivolumab
Nivolumab, a human monoclonal antibody that targets PD-1, was the first ICI approved for HCC (45). CheckMate-040 was a phase I/II dose-escalation and expansion study that overall demonstrated safety and efficacy of nivolumab in advanced HCC. Patients with Child-Pugh B7 disease, viral hepatitis (HBV or HCV), and previous sorafenib treatment were also included. In the dose-escalation phase, patients received 0.1 to 10 mg/kg of nivolumab every 2 weeks while those in the expansion phase were treated with 3 mg/kg every 2 weeks. Objective response rates were between 15-20% with median durations of response ranging from 9.9 to 17 months across both phases of the study. The median OS was 15 months in the dose-escalation phase, and even patients who had progressed on sorafenib achieved a median OS of 13.2 months. Most adverse events were manageable and comparable between those with Child-Pugh A and B disease (18, 46). These results led to the accelerated approval of nivolumab for second-line HCC treatment by the FDA in 2017 (45). This was later followed by the phase III CheckMate-459 trial that randomized 743 patients with advanced HCC to either nivolumab or sorafenib in the first-line setting. Although the primary endpoint of OS did not meet the predefined threshold of statistical significance (HR 0.83, p=0.0419), nivolumab showed a trend towards improved median OS (16.4 vs. 14.7 months; HR 0.85, p=0.0752) and ORR (15% vs. 7%) compared to sorafenib. Patients in the nivolumab arm also had fewer grade 3/4 treatment-related adverse events (22% vs. 49%) and lower rates of discontinuation (4% vs. 8%) (47). Based on these findings, the FDA did eventually withdraw accelerated approval of nivolumab, however it could be a potential option particularly for patients ineligible for TKIs or other anti-angiogenic agents and in those with Child-Pugh B per NCCN guidelines (15, 48).
Pembrolizumab
KEYNOTE-224 was a phase II trial that showed efficacy and tolerability of pembrolizumab in HCC previously treated with sorafenib (ORR 17%, 44% with stable disease), leading to FDA accelerated approval of the anti-PD-1 antibody as a second-line therapy option in 2018 (13, 49). Similar response rate was reported in the subsequent KEYNOTE-240, a phase III randomized control trial that assigned 413 patients on second-line treatment to best supportive care with or without pembrolizumab (200 mg every 3 weeks). Although pembrolizumab did not improve median OS (13.9 vs. 10.6 months, HR 0.78) and PFS (3 vs. 2.8 months) compared to placebo, ORR was significantly better (18.3% vs. 4.4%) with a considerable median duration of response of 13.8 months. The safety profile of pembrolizumab was manageable; grade 3 or higher immune-related adverse events occurred in 7.2% of patients and no flares of viral hepatitis were reported (50).
Combination Immune therapy
Nivolumab/Ipilimumab
One cohort of CheckMate-040 assessed the combination of nivolumab plus ipilimumab (anti-cytotoxic T-lymphocyte antigen-4 [CTLA-4]) in 148 cases of sorafenib-treated HCC. Patients were randomized to one of three arms composed of different doses and schedules of nivolumab plus ipilimumab. Across the three groups, ORR was 31% and eight patients had complete responses. Forty-nine patients in one arm received nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for 4 doses followed by nivolumab 240 mg every 2 weeks until disease progression or intolerability (14). This arm compared to the others had the best median OS at 22.8 months (vs. 12.5 and 12.7 months in the other groups) and disease control rate of 54% (vs. 43% and 49% in the other groups) (50). Responses were observed regardless of baseline PD-L1 status (14). The combination of nivolumab plus ipilimumab was soon approved by the FDA in 2020 for HCC after previous sorafenib treatment (51).
Atezolizumab/Bevacizumab
Bevacizumab, an anti-VEGF-A monoclonal antibody, has been evaluated as monotherapy and in combination with various cytotoxic agents for advanced HCC in multiple phase II studies (52–54). The landmark IMbrave150 trial demonstrated superiority of bevacizumab plus atezolizumab (anti-PD-L1) over sorafenib (12, 55). Prior to this trial, a phase 1b study had shown promising results from the combination, with an ORR of 36% and median PFS of 5.6 months while maintaining an acceptable toxicity profile (56). The IMbrave150 trial randomly assigned 501 patients to either atezolizumab plus bevacizumab (1200 mg and 15 mg/kg, respectively, every 3 weeks) or sorafenib (400 mg twice daily). This treatment combination compared to standard care significantly improved survival outcomes (HR 0.58, p<0.001) with higher 1-year OS rates (67.2% vs. 54.6%) and median PFS (6.8 vs. 4.3 months) (12). An updated analysis showed that survival benefit with the combination was sustained; median OS with atezolizumab plus bevacizumab was 19.2 months versus 13.4 months with sorafenib (HR 0.66, p=0.0009). The frequency of grade 3/4 adverse events was similar between both arms, with hypertension and proteinuria as the most common side effects of the combination treatment (12, 57). Importantly, analyses of patient-reported outcomes showed clinically meaningful improvements in reported quality of life, disease symptoms, and functioning with atezolizumab plus bevacizumab over sorafenib (58). The approval of this combination marked a significant shift in HCC treatment, with an immunotherapy-based regimen now recommended as the preferred first-line systemic therapy option based on improved survival (15).
Future Options
ICI Plus ICI Combinations
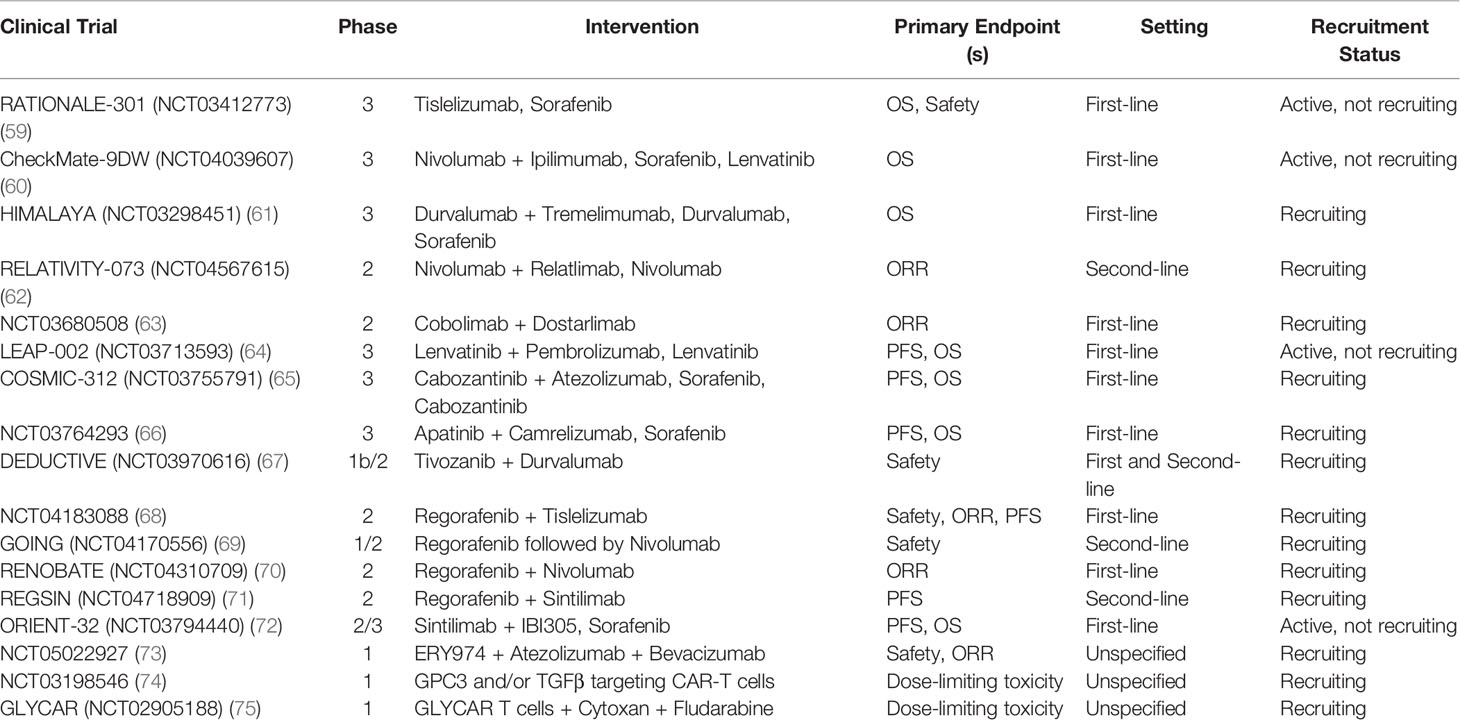
Promising results from ICI therapies in recent clinical trials have led to the investigation of other ICI-based combinations and immunotherapy strategies in advanced HCC (Table 2). The combination of different checkpoint inhibitors is appealing due to its potential to overcome resistance to single-agent immunotherapy by targeting multiple pathways the tumor uses to evade the immune response (28). After nivolumab plus ipilimumab demonstrated efficacy in sorafenib progressors, the anti-PD-1/CTLA-4 combination is currently being compared to sorafenib and cabozantinib as first-line treatment in CheckMate-9DW (NCT04039607) (14, 60). Durvalumab (anti-PD-L1) plus tremelimumab (anti-CTLA-4) is a similar ICI combination that showed significant clinical activity and tolerability for unresectable HCC after sorafenib in a phase I/II study (76). Recent presentation of the phase III HIMALAYA trial (NCT03298451) evaluating the STRIDE (Single T [tremelimumab] Regular Interval D [durvalumab]) regimen versus sorafenib demonstrated superior median OS (16.4 vs. 13.8 months, HR 0.78, p=0.0035) and ORR (20.1% vs. 5.1%) with comparable safety profiles. Additionally, single agent durvalumab was noninferior to sorafenib, with a median OS of 16.6 versus 13.8 months (HR 0.86, noninferiority margin 1.08) and ORR of 17%. Based on these results, dual ICI combination and single agent durvalumab may become potential first-line systemic therapy options for advanced HCC in the near future (61, 77). Other potential targets of checkpoint blockade include TIM-3 and LAG-3, which are both expressed along with PD-1 on CD8+ T cells and contribute to T cell dysfunction and suppression (78). Phase II trials of dual checkpoint blockade against PD-1 and LAG-3 (NCT04567615) as well as TIM-3 (NCT03680508) in HCC are ongoing (62, 63). Safety is a notable concern in combination immunotherapy, as shown in CheckMate-040 with up to 53% of patients on nivolumab plus ipilimumab developing grade 3/4 treatment-related adverse events (e.g. immune hepatitis, pneumonitis) (14). Therefore, patients should be monitored closely for toxicity while on therapy, and strategies for prevention and treatment of side effects should be further explored.
ICI Plus Anti-Angiogenesis Combinations
After the success of the IMbrave-150 trial, additional combinations of ICI plus anti-angiogenesis therapy (TKIs, mAb) are currently under development (12). Angiogenesis plays an important role in modulating the tumor microenvironment through inhibition of APCs and effectors cells as well as activation of inhibitory cells including Tregs and TAMs. Thus, the addition of anti-angiogenic agents to ICIs has the potential to boost the anti-tumor immune response and provides the basis for combination studies (79). KEYNOTE-524 was a phase Ib study that demonstrated promising activity of lenvatinib plus pembrolizumab with 46% ORR by mRECIST, median OS of 22 months, and manageable toxicity (80). Based on these results, the ongoing phase III LEAP-002 trial (NCT03713593) is evaluating lenvatinib with or without pembrolizumab in the first-line setting for unresectable HCC (64). The phase III COSMIC-312 trial (NCT03755791) of cabozantinib plus atezolizumab was recently announced by press release to improve PFS compared to sorafenib in untreated HCC at primary analysis, although the OS endpoint was missed and final results are pending (65, 81). Other ICI/anti-angiogenesis combinations such as apatinib plus camrelizumab (NCT03764293), tivozanib plus durvalumab (NCT03970616), and regorafenib plus tiselizumab (NCT04183088), are under current investigation in clinical trials (66–68).
Bispecific Antibodies
There are a variety of other immunotherapy-based approaches that are being evaluated in HCC besides ICIs. Bispecific antibodies are designed to recognize and bind specific tumor-associated antigens (e.g. AFP, GPC3) and effector cell receptors (e.g. CD3, CD28) in order to initiate tumor cytotoxicity (82). Ishiguro and colleagues successfully treated solid tumors in mouse models with a bispecific antibody (ERY974) combining CD3 on T cells and GPC3, which is expressed in most cases of HCC (83). Based on this preclinical data, a phase I study of ERY974 in combination with atezolizumab and bevacizumab in locally advanced or metastatic HCC is now ongoing (NCT05022927) (73). Other preclinical studies have demonstrated efficacy of bispecific antibodies targeting VEGF and EpCAM on HCC cancer cells in mice, providing basis for additional studies in humans (82).
Cancer Vaccines and Oncolytic Viruses
Vaccine therapy is another strategy to help enhance the anti-tumor immune response by using artifically designed tumor antigens (based on nucleic acids, peptides, or dendritic cells) to prime cytotoxic T cells (78). AFP-based vaccines were used in earlier trials and have since expanded to other tumor antigens such as GPC3 and hTERT (78, 84). Although studies of vaccines in HCC have generally had limited success, combining them with ICIs and other agents warrants further examination (78). Oncolytic viruses are designed to preferentially replicate in cancer cells and result in tumor cell lysis, causing release of tumor antigens that activate anti-tumor immune responses (28). The modified poxvirus Pexa-Vec (JX-594) is the most widely studied oncolytic virus in HCC and has shown clinical activity as well as tolerability in previous studies (85). PHOCUS was a phase III clinical trial evaluating Pexa-Vec followed by sorafenib versus sorafenib in advanced HCC, however the study was discontinued after interim analysis indicated the unlikelihood of meeting the OS primary endpoint (86, 87).
Adoptive Cell Therapy
Adoptive cell therapy is a method in which cells (e.g. NK cells, tumor-infiltrating lymphocytes, chimeric antigen receptor [CAR] T cells) are genetically engineered ex-vivo to target cancer cells and then reinfused into patients (88). Shi and colleagues discussed results from two phase I studies that enrolled 13 patients with advanced HCC treated with GPC3-CAR T cells; preliminary data showed 2 partial responses, 1-year OS rate of 42%, and most common toxicities included pyrexia, lymphopenia, and cytokine release syndrome (89). CAR T cell trials targeting other tumor antigens including EpCAM and TGF-β in addition to GPC3 are underway (74, 75, 90). We eagerly await the results from the aforementioned clinical trials.
Conclusion
Over the past decade, the treatment paradigm for advanced HCC has dramatically changed. Molecular targeted agents represented the first important advancement in HCC treatment after a long history of ineffective cytotoxic chemotherapy. Despite this progress, long-term survival of patients with advanced stage disease remains low, highlighting the need for further improvement of therapy. In recent years, immunotherapy has changed the landscape of cancer treatment. Interest in its application for HCC has significantly grown given long lasting benefits seen in a small subset of patients, which propelled several studies evaluating other ICI combinations and novel modalities of immunotherapy. Further research will be needed to identify biomarkers to determine which patients will benefit from immunotherapy and establish the safety and efficacy in those with poor liver function, especially given their very limited treatment options. With more patients receiving ICI-based frontline therapy, future studies will also need to determine sequencing of treatments after immunotherapy exposure and investigate managing immunotherapy resistance and toxicity.
Author Contributions
SK, LK, and MA: study conception, design, and drafting of manuscript. SK and LK: acquisition of data. SK and MA: analysis and interpretation of data. SK, LK, BFE-R, and MA: critical revision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Singal AG, Lampertico P, Nahon P. Epidemiology and Surveillance for Hepatocellular Carcinoma: New Trends. J Hepatol (2020) 72:250–61. doi: 10.1016/j.jhep.2019.08.025
3. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The Economic and Clinical Burden of Nonalcoholic Fatty Liver Disease in the United States and Europe. Hepatology (2016) 64:1577–86. doi: 10.1002/hep.28785
4. Llovet JM, Kelley RK, Villaneuva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular Carcinoma. Nat Rev Dis Prim (2021) 7:6. doi: 10.1038/s41572-020-00240-3
5. Villaneuva A. Hepatocellular Carcinoma. N Engl J Med (2019) 380:1450–62. doi: 10.1056/NEJMra1713263
6. Thomas MB, O’Beirne JP, Furuse J, Chan ATC, Abou-Alfa G, Johnson P. Systemic Therapy for Hepatocellular Carcinoma: Cytotoxic Chemotherapy, Targeted Therapy and Immunotherapy. Ann Surg Onc (2008) 15:1008–14. doi: 10.1245/s10434-007-9705-0
7. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blacn JF, et al. Sorafenib in Advanced Hepatocellularcarcinoma. N Engl J Med (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
8. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib Versus Sorafenib in First-Line Treatment of Patients With Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
9. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for Patients With Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (2017) 389:56–66. doi: 10.1016/S0140-6736(16)32453-9
10. Farkona S, Diamandis EP, Blasutig IM. Cancer Immunotherapy: The Beginning of the End of Cancer? BMC Med (2016) 14:73. doi: 10.1186/s12916-016-0623-5
11. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey L, et al. Overall Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2017) 377:1345–56. doi: 10.1056/NEJMoa1709684
12. Finn RS, Win S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
13. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib (KEYNOTE-224): A Non-Randomised, Open-Label Phase 2 Trial. Lancet Oncol (2018) 19:940–52. doi: 10.1016/S1470-2045(18)30351-6
14. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol (2020) 6:e204564. doi: 10.1001/jamaoncol.2020.4564
15. National Comprehensive Cancer Network. Hepatobiliary Cancers (Version 5.2021) (2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (Accessed November 10, 2021).
16. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in Patients With Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med (2018) 379:54–63. doi: 10.1056/NEJMoa1717002
17. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab After Sorafenib in Patients With Advanced Hepatocellular Carcinoma and Increased α-Fetoprotein Concentrations (REACH-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol (2019) 20:282–96. doi: 10.1016/S1470-2045(18)30937-9
18. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in Patients With Advanced Hepatocellular Carcinoma (CheckMate 040): An Open-Label, Non-Comparative, Phase 1/2 Dose Escalation and Expansion Trial. Lancet (2017) 389:2492–502. doi: 10.1016/S0140-6736(17)31046-2
19. Jenne CJ, Kubes P. Immune Surveillance by the Liver. Nat Immunol (2013) 14:996–1006. doi: 10.1038/ni.2691
20. Shetty S, Lalor PF, Adams DH. Liver Sinusoidal Endothelial Cells—Gatekeepers of Hepatic Immunity. Nat Rev Gastroenterol Hepatol (2018) 15:555–67. doi: 10.1038/s41575-018-0020-y
21. Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer Cells in the Liver. Compr Physiol (2013) 3:785–97. doi: 10.1002/cphy.c120026
22. Breous E, Somanathan S, Vandenberghe LH, Wilson JM. Hepatic Regulatory T Cells and Kupffer Cells are Crucial Mediators of Systemic T Cell Tolerance to Antigens Targeting Murine Liver. Hepatology (2009) 50:612–21. doi: 10.1002/hep.23043
23. Dou L, Ono Y, Chen YF, Thomson AW, Chen XP. Hepatic Dendritic Cells, the Tolerogenic Liver Environment, and Liver Disease. Semin Liver Dis (2018) 38:170–80. doi: 10.1055/s-0038-1646949
24. Oura K, Morishita A, Tani J, Masaki T. Tumor Immune Microenvironment and Immunosuppressive Therapy in Hepatocellular Carcinoma: A Review. Int J Mol Sci (2021) 22:5801. doi: 10.3390/ijms22115801
25. Zhou J, Wang W, Li Q. Potential Therapeutic Targets in the Tumor Microenvironment of Hepatocellular Carcinoma: Reversing the Protumor Effect of Tumor-Associated Macrophages. J Exp Clin Cancer Res (2021) 40:73. doi: 10.1186/s13046-021-01873-2
26. Greten TF, Wang XW, Korangy F. Current Concepts of Immune Based Treatments for Patients With HCC: From Basic Science to Novel Treatment Approaches. Gut (2015) 64:842–48. doi: 10.1136/gutjnl-2014-307990
27. Ilkovitch D, Lopez DM. The Liver is a Site for Tumor Induced Myeloid-Derived Suppressor Cell Accumulation and Immunosuppression. Cancer Res (2009) 69:5514–21. doi: 10.1158/0008-5472.CAN-08-4625
28. Prieto J, Melero I, Sangro B. Immunological Landscape and Immunotherapy of Hepatocellular Carcinoma. Nat Rev Gastroenterol Hepatol (2015) 12:681–700. doi: 10.1038/nrgastro.2015.173
29. Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, et al. PD-1 and PD-L1 Upregulation Promotes CD8(+) T-Cell Apoptosis and Postoperative Recurrence in Hepatocellular Carcinoma Patients. Int J Cancer (2011) 128:887–96. doi: 10.1002/ijc.25397
30. Ma J, Zheng B, Goswami S, Meng L, Zhang D, Cao C, et al. PD1 Hi CD8 + T Cells Correlate With Exhausted Signature and Poor Clinical Outcome in Hepatocellular Carcinoma. J Immunother Cancer (2019) 7:331. doi: 10.1186/s40425-019-0814-7
31. Matsui M, Machia S, Itani-Yohda T, Akatsuka T. Downregulation of the Proteasome Subunits, Transporter, and Antigen Presentation in Hepatocellular Carcinoma, and Their Restoration by Interferon-Gamma. J Gastroenterol Hepatol (2002) 17:897–907. doi: 10.1046/j.1440-1746.2002.02837.x
32. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region With Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol (2009) 10:25–34. doi: 10.1016/S1470-2045(08)70285-7
33. Marrero JA, Kudo M, Venook AP, Ye SL, Bronowicki JP, Chen XP, et al. Observational Registry of Sorafenib Use in Clinical Practice Across Child-Pugh Subgroups: The GIDEON Study. J Hepatol (2016) 65:1140–7. doi: 10.1016/j.jhep.2016.07.020
34. FDA Expands Approved Use of Stivarga to Treat Liver Cancer, in: Food and Drug Administration Web Site (2017). Available at: https://www.fda.gov/news-events/press-announcements/fda-expands-approved-use-stivarga-treat-liver-cancer (Accessed November 14, 2021).
35. Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, et al. Sunitinib Versus Sorafenib in Advanced Hepatocellular Cancer: Results of a Randomized Phase III Trial. J Clin Oncol (2013) 31:4067–75. doi: 10.1200/JCO.2012.45.8372
36. Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, et al. Brivanib Versus Sorafenib as First-Line Therapy in Patients With Unresectable, Advanced Hepatocellular Carcinoma: Results From the Randomized Phase III BRISK-FL Study. J Clin Oncol (2013) 31:3517–24. doi: 10.1200/JCO.2012.48.4410
37. Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, et al. Linifanib Versus Sorafenib in Patients With Advanced Hepatocellular Carcinoma: Results of a Randomized Phase III Trial. J Clin Oncol (2015) 33:172–9. doi: 10.1200/JCO.2013.54.3298
38. Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, et al. SEARCH: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial of Sorafenib Plus Erlotinib in Patients With Advanced Hepatocellular Carcinoma. J Clin Oncol (2015) 33:559–66. doi: 10.1200/JCO.2013.53.7746
39. FDA Approves Lenvatinib for Unresectable Hepatocellular Carcinoma, in: Food and Drug Administration Web Site (2018). Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-unresectable-hepatocellular-carcinoma (Accessed November 14, 2021).
40. Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda M, Okusaka T, et al. Phase 2 Study of Lenvatinib in Patients With Advanced Hepatocellular Carcinoma. J Gastroenterol (2017) 52:512–9. doi: 10.1007/s00535-016-1263-4
41. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Cheng AL, et al. Analysis of Survival and Objective Response (OR) in Patients With Hepatocellular Carcinoma in a Phase III Study of Lenvatinib (REFLECT). J Clin Oncol (2019) 37:186. doi: 10.1200/JCO.2019.37.4_suppl.186
42. Kelley RK, Meyer T, Rimassa L, Merle P, Park JW, Yau T, et al. Serum Alpha-Fetoprotein Levels and Clinical Outcomes in the Phase III CELESTIAL Study of Cabozantinib Versus Placebo in Patients With Advanced Hepatocellular Carcinoma. Clin Cancer Res (2020) 26:4795–804. doi: 10.1158/1078-0432.CCR-19-3884
43. Zhu AX, Finn RS, Mulcahy M, Gurtler J, Sun W, Schwartz JD, et al. A Phase II and Biomarker Study of Ramucirumab, a Human Monoclonal Antibody Targeting the VEGF Receptor-2, as First-Line Monotherapy in Patients With Advanced Hepatocellular Cancer. Clin Cancer Res (2013) 19:6614–23. doi: 10.1158/1078-0432.CCR-13-1442
44. Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Ramucirumab Versus Placebo as Second-Line Treatment in Patients With Advanced Hepatocellular Carcinoma Following First-Line Therapy With Sorafenib (REACH): A Randomised, Double-Blind, Multicentre, Phase 3 Trial. Lancet Oncol (2015) 16:859–70. doi: 10.1016/S1470-2045(15)00050-9
45. FDA Grants Accelerated Approval to Nivolumab for HCC Previously Treated With Sorafenib, in: Food and Drug Administration Web Site (2017). Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib (Accessed November 14, 2021).
46. Kudo M, Matilla A, Santoro A, Melero I, Gracian AC, Acosta-Rivera M, et al. Checkmate-040: Nivolumab (NIVO) in Patients (Pts) With Advanced Hepatocellular Carcinoma (aHCC) and Child-Pugh B (CPB) Status. J Clin Oncol (2019) 37(suppl):4. doi: 10.1200/JCO.2019.37.4_suppl.327
47. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. CheckMate 459: A Randomized, Multi-Center Phase III Study of Nivolumab (NIVO) vs. Sorafenib (SOR) as First-Line (1L) Treatment in Patients (Pts) With Advanced Hepatocellular Carcinoma (aHCC). Ann Oncol (2019) 30:874–75. doi: 10.1093/annonc/mdz394.029
48. FDA Committee Votes to Withdraw Two Accelerated Approvals of Cancer Treatments, In: ASH Clinical News (2021). Available at: https://www.ashclinicalnews.org/online-exclusives/fda-committee-votes-withdraw-two-accelerated-approvals-cancer-treatments/ (Accessed November 16, 2021).
49. FDA Grants Accelerated Approval to Pembrolizumab for Hepatocellular Carcinoma, in: Food and Drug Administration Web Site (2018). Available at: https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma (Accessed November 14, 2021).
50. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol (2020) 38:193–202. doi: 10.1200/JCO.19.01307
51. FDA Grants Accelerated Approval to Nivolumab and Ipilimumab Combination for Hepatocellular Carcinoma, In: Food and Drug Administration Web Site (2020). Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-and-ipilimumab-combination-hepatocellular-carcinoma (Accessed November 14, 2021).
52. Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, et al. Phase II Trial Evaluating the Clinical and Biologic Effects of Bevacizumab in Unresectable Hepatocellular Carcinoma. J Clin Oncol (2008) 26:2992–8. doi: 10.1200/JCO.2007.15.9947
53. Hsu CH, Yang TS, Hsu C, Toh HC, Epstein RJ, Hsiao LT, et al. Efficacy and Tolerability of Bevacizumab Plus Capecitabine as First-Line Therapy in Patients With Advanced Hepatocellular Carcinoma. Br J Cancer (2010) 102:981–6. doi: 10.1038/sj.bjc.6605580
54. Zhu AX, Blaszkowsky S, Ryan DP, Clark JW, Muzikansky A, Horgan K, et al. Phase II Study of Gemcitabine and Oxaliplatin in Combination With Bevacizumab in Patients With Advanced Hepatocellular Carcinoma. J Clin Oncol (2006) 24:1898–903. doi: 10.1200/JCO.2005.04.9130
55. FDA Approves Atezolizumab Plus Bevacizumab for Unresectable Hepatocellular Carcinoma, In: Food and Drug Administration Web Site (2020). Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-plus-bevacizumab-unresectable-hepatocellular-carcinoma (Accessed November 14, 2021).
56. Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. Atezolizumab With or Without Bevacizumab in Unresectable Hepatocellular Carcinoma (GO30140): An Open-Label, Multicentre, Phase 1b Study. Lancet Oncol (2020) 6:808–20. doi: 10.1016/S1470-2045(20)30156-X
57. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. IMbrave150: Updated Overall Survival (OS) Data From a Global, Randomized, Open-Label Phase III Study of Atezolizumab (Atezo) + Bevacizumab (Bev) Versus Sorafenib (Sor) in Patients (Pts) With Unresectable Hepatocellular Carcinoma (HCC). J Clin Oncol (2021) 39:267. doi: 10.1200/JCO.2021.39.3_suppl.267
58. Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, et al. Patient-Reported Outcomes With Atezolizumab Plus Bevacizumab Versus Sorafenib in Patients With Unresectable Hepatocellular Carcinoma (IMbrave150): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol (2021) 22:991–1001. doi: 10.1016/S1470-2045(21)00151-0
59. Phase 3 Study of Tislelizumab Versus Sorafenib in Participants With Unresectable HCC, in: ClinicalTrials.gov Identifier: NCT03412773 (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT03412773 (Accessed November 20, 2021).
60. A Study of Nivolumab in Combination With Ipilimumab in Participants With Advanced Hepatocellular Carcinoma (CheckMate 9dw), in: ClinicalTrials.gov Identifier: NCT04039607 (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT04039607 (Accessed November 19, 2021).
61. Abou-Alfa GK, Chan SL, Furuse J, Galle PR, Kelley RK, Qin S, et al. A Randomized, Multicenter Phase 3 Study of Durvalumab (D) and Tremelimumab (T) as First-Line Treatment in Patients With Unresectable Hepatocellular Carcinoma (HCC): HIMALAYA Study. J Clin Oncol (2018) 36(suppl):S15. doi: 10.1200/JCO.2018.36.15_suppl.TPS414
62. Sangro B, Numata K, Huang Y, Gomez-Martin C, Hiraoka A, Moriguchi M, et al. P-61 Relatlimab + Nivolumab in Patients With Advanced Hepatocellular Carcinoma Who Are Naive to Immuno-Oncology Therapy But Progressed on Tyrosine Kinase Inhibitors, a Phase 2, Randomized, Open-Label Study: RELATIVITY-073. Ann Oncol (2021) 32:S117. doi: 10.1016/j.annonc.2021.05.116
63. TSR-022 (Anti-TIM-3 Antibody) and TSR-042 (Anti-PD-1 Antibody) in Patients With Liver Cancer. In: ClinicalTrials.gov Identifier: NCT03680508. Available at: https://clinicaltrials.gov/ct2/show/NCT03680508.
64. Llovet JM, Kudo M, Cheng AL, Finn RS, Galle PR, Kaneko S, et al. Lenvatinib (Len) Plus Pembrolizumab (Pembro) for the First-Line Treatment of Patients (Pts) With Advanced Hepatocellular Carcinoma (HCC): Phase 3 LEAP-002 Study. J Clin Oncol (2019) 37(suppl):15. doi: 10.1200/JCO.2019.37.15_suppl.TPS4152
65. Kelley RK, Oliver JW, Hazra S, Benzaghou F, Yau T, Cheng AL, et al. Cabozantinib in Combination With Atezolizumab Versus Sorafenib in Treatment-Naive Advanced Hepatocellular Carcinoma: COSMIC-312 Phase III Study Design. Future Oncol (2020) 16:1525–36. doi: 10.2217/fon-2020-0283
66. Su J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in Combination With Apatinib in Patients With Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-Label, Phase II Trial. Clin Cancer Res (2021) 27:1003–11. doi: 10.1158/1078-0432.CCR-20-2571
67. A Study of Tivozanib in Combination With Durvalumab in Subjects With Advanced Hepatocellular Carcinoma (DEDUCTIVE), In: ClinicalTrial.gov Identifier: NCT03970616 (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT03970616 (Accessed November 19, 2021).
68. Regorafenib Plus Tislelizumab as First-Line Systemic Therapy for Patients With Advanced Hepatocellular Carcinoma, In: ClinicalTrials.gov Identifier: NCT04183088 (2020). Available at: https://www.clinicaltrials.gov/ct2/show/NCT04183088 (Accessed November 19, 2021).
69. Regorafenib Followed by Nivolumab in Patients With Hepatocellular Carcinoma (GOING), In: ClinicalTrials.gov Identifier: NCT04170556 (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT04170556 (Accessed November 20, 2021).
70. Combination of Regorafenib and Nivolumab in Unresectable Hepatocellular Carcinoma (RENOBATE), In: ClinicalTrials.gov Identifier: NCT04310709 (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT04310709 (Accessed November 20, 2021).
71. Regorafenib Plus Sintilimab vs. Regorafenib as the Second-Line Treatment for HCC (REGSIN), In: ClinicalTrials.gov Identifier: NCT04718909 (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT04718909 (Accessed November 20, 2021).
72. A Study to Evaluate the Efficacy and Safety of Sintilimab in Combination With IBI305 (Anti-VEGF Monoclonal Antibody) Compared to Sorafenib as the First-Line Treatment for Advanced Hepatocellular Carcinoma, in: ClinicalTrials.gov Identifier: NCT03794440 (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT03794440 (Accessed November 20, 2021).
73. A Phase I Study of ERY974 in Patients With Hepatocellular Carcinoma, In: ClinicalTrials.gov Identifier: NCT05022927 (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT05022927 (Accessed November 15, 2021).
74. GPC3-CAR-T Cells for Immunotherapy of Cancer With GPC3 Expression, in: ClinicalTrials.gov Identifier: NCT03198546 (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT03198546 (Accessed November 15, 2021).
75. Glypican 3-Specific Chimeric Antigen Receptor Expressing T Cells for Hepatocellular Carcinoma (GLYCAR), In: ClinicalTrials.gov Identifier: NCT0290518 (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT02905188 (Accessed November 15, 2021).
76. Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J Clin Oncol (2021) 39:2991–3001. doi: 10.1200/JCO.20.03555
77. About-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 Randomized, Open-Label, Multi-Center Study of Tremelimumab (T) and Durvalumab (D) as First-Line Therapy in Patients (pts) With Unresectable HepatoCellular Carcinoma (uHCC): HIMALAYA. J Clin Oncol (2022) 40(suppl 4):379. doi: 10.1200/JCO.2022.40.4_suppl.379
78. Johnston MP, Khakoo SI. Immunotherapy for Hepatocellular Carcinoma: Current and Future. World J Gastroenterol (2019) 25:2977–89. doi: 10.3748/wjg.v25.i24.2977
79. Rahma OE, Hodi FS. The Intersection Between Tumor Angiogenesis and Immune Suppression. Clin Cancer Res (2019) 25:5449–57. doi: 10.1158/1078-0432.CCR-18-1543
80. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
81. Exelixis and Ipsen Announce Cabozantinib in Combination With an Immune Checkpoint Inhibitor Significantly Improved Progression-Free Survival in Phase 3 COSMIC-312 Pivotal Trial in Patients With Previously Untreated Advanced Liver Cancer, In: Exelixis (2021). Available at: https://ir.exelixis.com/news-releases/news-release-details/exelixis-and-ipsen-announce-cabozantinib-combination-immune (Accessed November 19, 2021).
82. Hoseini SS, Cheung NV. Immunotherapy of Hepatocellular Carcinoma Using Chimeric Antigen Receptors and Bispecific Antibodies. Cancer Lett (2017) 399:44–52. doi: 10.1016/j.canlet.2017.04.013
83. Ishiguro T, Sano Y, Komatsu SI, Kamata-Sakurai M, Kaneko A, Kinoshita Y, et al. An Anti-Glypican 3/CD3 Bispecific T Cell-Redirecting Antibody for Treatment of Solid Tumors. Sci Transl Med (2017) 9:eaal4291. doi: 10.1126/scitranslmed.aal4291
84. Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, et al. T-Cell Responses to HLA-A*0201 Immunodominant Peptides Derived From Alpha-Fetoprotein in Patients With Hepatocellular Cancer. Clin Cancer Res (2003) 9:5902–08.
85. Heo J, Breitbach C, Cho M, Hwang TH, Kim CW, UB J, et al. A Phase II Trial of JX-594, a Targeted Multimechanistic Oncolytic Vaccinia Virus, Followed by Sorafenib in Patients With Advanced Hepatocellular Carcinoma (HCC). J Clin Oncol (2012) 30(suppl):15. doi: 10.1200/jco.2012.30.15_suppl.e14566
86. Abou-Alfa GK, Galle PR, Chao Y, Brown KT, Heo J, Borad MJ, et al. PHOCUS: A Phase 3 Randomized, Open-Label Study Comparing the Oncolytic Immunotherapy Pexa-Vec Followed by Sorafenib (SOR) vs SOR in Patients With Advanced Hepatocellular Carcinoma (HCC) Without Prior Systemic Therapy. J Clin Oncol (2016) 34(suppl):15. doi: 10.1200/JCO.2016.34.15_suppl.TPS4146
87. SillaJen Announces Conclusions From Interim Futility Analysis of Phase 3 PHOCUS Trial in HCC, In: BioSpace (2019). Available at: https://www.biospace.com/article/releases/sillajen-announces-conclusions-from-interim-futility-analysis-of-phase-3-phocus-trial-in-hcc/ (Accessed November 19, 2021).
88. Rosenberg SA, Restifo NP. Adoptive Cell Transfer as Personalized Immunotherapy for Human Cancer. Science (2015) 348:62–8. doi: 10.1126/science.aaa4967
89. Shi D, Shi Y, Kaseb AO, Qi X, Zhang Y, Chi J, et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin Cancer Res (2020) 26:3979–89. doi: 10.1158/1078-0432.CCR-19-3259
90. A Clinical Research of CAR T Cells Targeting EpCAM Positive Cancer (CARTEPC), In: ClinicalTrials.gov Identifier: NCT03013712 (2017). Available at: https://clinicaltrials.gov/ct2/show/NCT03013712 (Accessed November 15, 2021).
Keywords: hepatocellular carcinoma, immunotherapy, tumor microenvironment, immune checkpoint inhibitor, molecular targeted agents
Citation: Kang SM, Khalil L, El-Rayes BF and Akce M (2022) Rapidly Evolving Landscape and Future Horizons in Hepatocellular Carcinoma in the Era of Immuno-Oncology. Front. Oncol. 12:821903. doi: 10.3389/fonc.2022.821903
Received: 25 November 2021; Accepted: 08 March 2022;
Published: 31 March 2022.
Edited by:
Maen Abdelrahim, Houston Methodist Research Institute, United StatesReviewed by:
Tibor Bakacs, Alfred Renyi Institute of Mathematics, HungaryTommaso Stecca, ULSS2 Marca Trevigiana, Italy
Copyright © 2022 Kang, Khalil, El-Rayes and Akce. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehmet Akce, mehmet.akce@emory.edu
 Sandra Mirie Kang
Sandra Mirie Kang Lana Khalil
Lana Khalil Mehmet Akce
Mehmet Akce